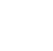4. Monitoring
4.1 HCB Monitoring
Monitoring is a process of routinely observing or measuring something through space and time, resulting in the accumulation of data. In the case of cyanobacteria monitoring, observations and measurements usually evaluate the presence or amount of cyanobacteria and whether cyanotoxins are present. Other less common monitoring approaches may involve evaluating aquatic organism community composition, relative abundance and dominance, cyanobacteria cell counts, fluorometric measurements, genetic techniques, and cyanotoxin variants. This Section focuses on monitoring approaches that directly measure cyanobacteria and cyanotoxins.
Cyanobacteria monitoring parameters can be selectively implemented to serve many purposes, such as guiding outreach and education, tracking HCB progression and trends, meeting regulatory requirements, or driving decision-making processes and management actions. These activities can have one or more specific end goals, such as informing and motivating stakeholders, improving water quality, protecting environmental and public health, and preventing HCBs. For more information on monitoring as part of your HCB response plan, see Section 5.1.2 of our communication and response planning Section.
Time and personnel needed to monitor for HCBs can be considerable. Methods should be selected carefully to ensure that they are implemented effectively and achieve your intended outcomes and goals. A tiered approach to monitoring may be effective, depending on the level of cyanobacteria concern. A simple, one-time water sample may be all that is needed to introduce local stakeholders to the cyanobacteria in their water body, jumpstarting education and outreach activities; however, to better understand the local lake ecosystem and identify management approaches, consistent long-term data are necessary.
Monitoring to discern trends through time and track conditions leading up to bloom formation requires extensive resources and sound planning. At any level, a plan to determine what, where, when, and how samples should be collected is necessary. Developing partnerships and drawing in local resources facilitates data sharing, improves coordination, and allows development of a larger monitoring program than might otherwise be possible.
Click here to access the Monitoring Tool.
4.2 Developing a Cyanobacteria Monitoring Program
A successful monitoring program always begins with planning and developing a monitoring strategy. Identifying your desired end goals or objectives and understanding cyanobacteria ecology will help define the necessary steps and methods that should be used.
Monitoring during HCB events provides information about current conditions and is often a reactive approach. Determining how current conditions compare to previous bloom events and non-bloom periods requires a proactive approach. Therefore, it is important to consider how monitoring should be conducted before, during, and after HCBs.
Comprehensive monitoring can become very expensive if the area monitored experiences frequent and widespread blooms. While the cost of a comprehensive cyanobacteria monitoring program can be high, degradation of the water resource has significant human and economic impacts (see Section 3). Continuing to use a management strategy that does not produce the expected results can also have significant impacts. A good monitoring plan supports efficient use of resources to meet many management goals.
When designing a monitoring program, consider how it will inform and support your management strategies and HCB response activities. How will the data convey information about bloom severity to stakeholders, residents, and visitors? It is also helpful to evaluate how well stakeholders processed and responded to your monitoring and outreach about your data. This will improve future responses to HCBs and inform management and prevention recommendations (see Section 5.2.5).
We encourage you to read Section 5 to assist you in developing a monitoring program that aligns with your cyanobacteria response goals. Important considerations when designing an effective and sustainable HCB monitoring program include:
- Purpose: Monitoring programs can provide information on current conditions, variability across space and time, and insight to long-term change. They generate critical information for public health response and serve as educational resources for the general public. As you select a monitoring method, consider what kind of data it produces and how the data will support your needs. If you plan to only monitor during HCBs, then your design will likely be very different from a program tracking water body changes before, during, and after an HCB occurs.
- Location: HCBs are not uniformly present throughout a water body, and one location rarely is representative of conditions elsewhere. This is true for both planktonic and benthic cyanobacteria (Figures 3-2, 4-1, and 4-2, see also Section 3). Addressing drinking water and recreation needs, for example, may require sampling near a water treatment plant intake or a swimming beach, respectively. In lakes, downwind or shoreline locations often exhibit high concentrations of planktonic cyanobacteria when compared to open water locations. Distribution across the water surface and with depth changes rapidly with wind and weather conditions. Monitoring approaches in flowing waters, such as rivers and streams, will differ from those used in lakes, reservoirs, and ponds. Benthic mats can be found in lakes or ponds and flowing waters. Such variability means that full characterization of a water body will require multiple sampling locations and often the use of more than one sampling method. See Section 3 of HCB-2, Monitoring for benthic cyanobacteria.
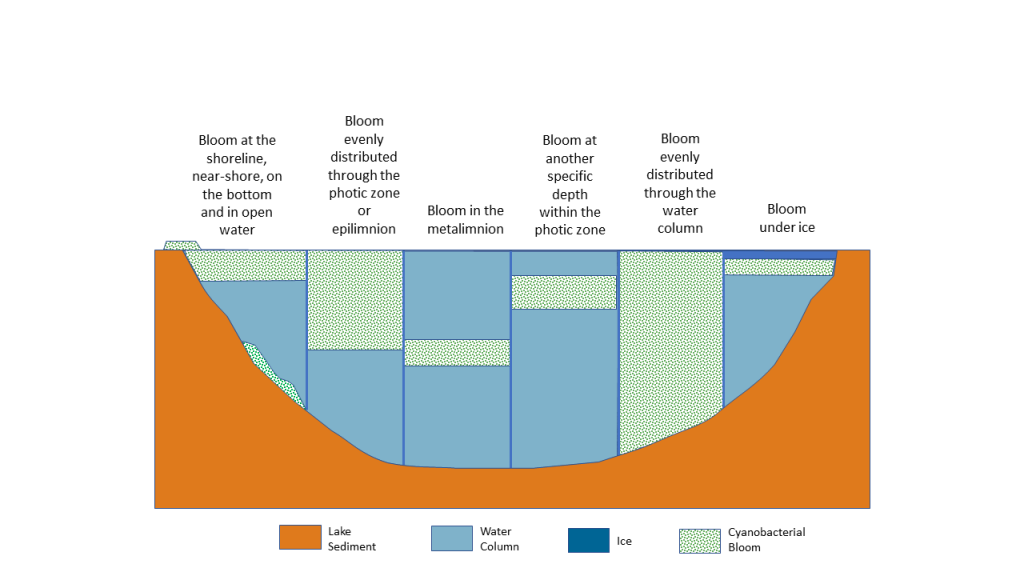
Figure 4‑1. Examples of cyanobacteria distribution in a lake or pond. In addition to planktonic species, many lakes and ponds have benthic species as well.
Source: Modified from Graham et al. (2008). Used with permission.
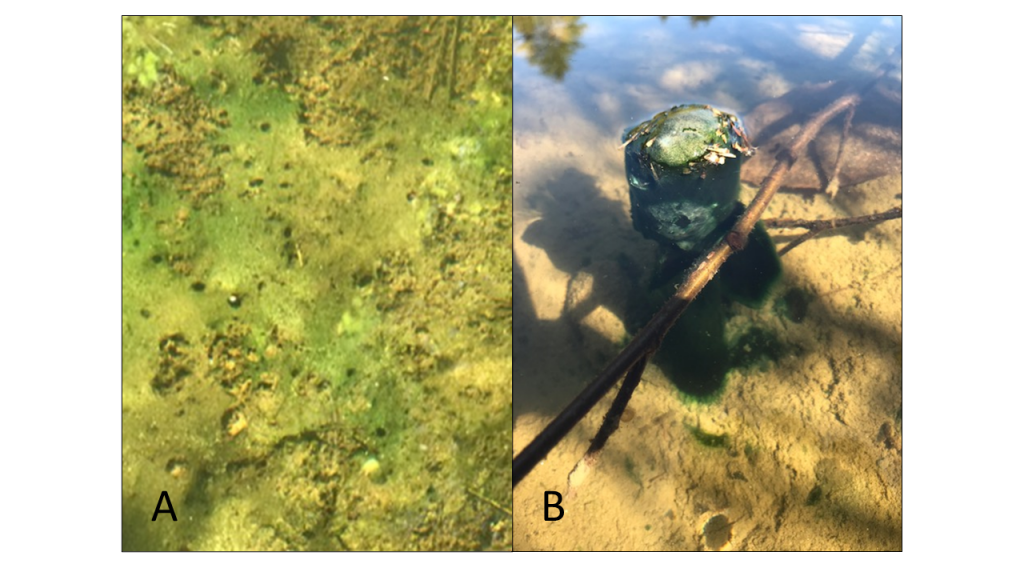
Figure 4‑2. Common appearance and distribution of benthic cyanobacteria, which may be attached to substrate (A) or may break free as mats and float to the surface (B).
Source: New Hampshire Department of Environmental Services (A) and Vermont Department of Environmental Conservation (B). Used with permission.
- Time of day: Like many organisms, cyanobacteria exhibit diurnal cycles that can influence data interpretation. For example, HCBs can be strongly responsive to sunlight under certain water conditions, accumulating at the surface during midday hours but dispersing as sunlight wanes. You should record the time of sample collection for all your samples. When evaluating trends over time, you should collect samples at approximately the same time of day to avoid bias that may be introduced, for example, by comparing morning cyanobacteria densities to those found in the afternoon.
- Response time: Sample collection and analysis take time. Your sampling approach and choice of method will determine how long it takes to get results. By the time results are available, cyanobacteria conditions may have changed. This is an important consideration when incorporating monitoring into your response and communication plans. The U.S. Geological Survey office in Kansas has several time-lapse videos that illustrate this phenomenon.
- Type of analyses: You should choose the specific types of data to be collected based on your program objectives. With each type comes an associated cost, some level of analytical expertise, and a minimum time for analysis and turnaround. You should consider how the data will be summarized, visualized, and presented. Some types of analyses may support management decisions, illuminate bloom processes, or meet your objectives better than others.
- Quality assurance: Ensuring the quality of the data you collect will produce data that are useful, defensible, and cost-effective. High-quality data can be used with confidence in the decision-making process and give your program solid footing from start to finish. USEPA has developed several guidance documents that help groups build a quality assurance project plan (QAPP) and help ensure that key aspects of the process are not overlooked (USEPA 2020a, c). Every monitoring program should have a QAPP.
- Sustainability: As with any monitoring program, sustainability is important. You should think about strategies that will ensure that the program can be maintained to meet your objectives over time, especially where funding and supporting staff may be scarce. Your plan may include building in-house expertise and phasing in program growth, or it may be limited to essential parameters only. Having the right people at the table as you develop a monitoring strategy will be critical to its success and its sustainability.
- People: Public interest and support is important when establishing HCB monitoring strategies. Individuals with in-depth knowledge and monitoring experience can provide valuable insights to appropriate sampling parameters and approaches to get started. Those with less experience but a strong interest in the water body can provide assistance and ensure that the program remains sustainable well into the future. Partnerships can successfully leverage available expertise, funding, and staffing to build a monitoring program. Government agencies (federal, state, municipal), universities, lake or watershed associations, and residents are all stakeholders in cyanobacteria management and potential partners in your monitoring program.
- Consistency: Before you begin to document blooms, determine what parameters and measurements are needed for assessment. Everyone—citizen groups, local boards of health, and state officials—should use the same methods whenever possible to determine if a bloom is present and to evaluate changes in a bloom. For consistency, make sure that everyone has reviewed the methods and followed steps outlined in the protocol. This will make your data more useful to everyone and may provide greater clarity about what is occurring in the water body.
- Jurisdiction and coordination: More than one entity may have jurisdiction over a water body, and these entities may be required to conduct monitoring to meet specific requirements. Drinking water facilities, for example, often have their own cyanobacteria monitoring and response plans. Coordination with local stakeholders reduces redundancy in monitoring, identifies gaps, and can bring all available monitoring data together in one place to support HCB response.
4.3 Approaches to Monitoring
Monitoring programs often combine two or more sampling methods. Your cyanobacteria response plan (Section 5) outlines how you will respond to HCBs and the action levels you will use. Qualitative approaches (for example, photographs and other methods that do not provide a numerical result) may provide a rapid first assessment and enable you to quickly take specific actions, such as closing a beach. Next steps often include collecting samples that will take longer to analyze and return a quantifiable result that informs your next steps, such as deciding how long the beach must remain closed (Figure 4-3).
Figure 4‑3. Common sequence of monitoring steps used to evaluate risk from cyanobacteria exposure. Your cyanobacteria response plan outlines how analytical data are used to determine next steps (see Figure 5.2, Idaho’s HCB Response Plan)
Source: ITRC. Used with permission.
The material we present in this Section is divided into methods that provide information on cyanobacteria abundance and those that focus on cyanotoxins. We developed a set of criteria (see the read more option below) and an interactive selection tool to help you review and select methods suited to your goals based on characteristics such as turnaround time and level of expertise required.
4.3.1 Methods to Evaluate Cyanobacteria Abundance
Table 4-1 provides an overview of sampling and assessment methods suitable to determine cyanobacteria abundance and taxonomic composition. For a more complete description of each method, use the hyperlink to access its Section in our document. You can also use our interactive tool to explore monitoring methods.
Table 4‑1. Monitoring methods used to identify and enumerate cyanobacteria
| Methods for Identification and Enumeration | Result Type | Turnaround Time |
| Visual Assessments | Qualitative | <1 day |
| Jar and Stick Tests | Qualitative | <1 day |
| Pigments | Quantitative | <1 day to 3 days (varies by method) |
| Remote Sensing | Qualitative/quantitative | <1 day to 1 day (varies by platform) |
| Microscopy | Qualitative/quantitative | <1 day |
| Genetic Methods for Identification | Quantitative | <1 day |
| Semi-automated Classification and Machine Learning | Quantitative | <1 day |
4.3.1.1 Visual Assessments
- Result: Qualitative
- Sampling type: Variable
- Turnaround time: <1 day
- Level of training: Novice
- Laboratory required: No
- Relative cost: $
- Cyanobacteria presence/absence: Suitable
- Cyanobacteria identification: Not suitable
- Cyanobacteria density: Not suitable
- Cyanotoxin presence/absence: Not suitable
Visual assessments consist of observing an area of a water body where water is colored differently than other areas or where surface scum is present and then comparing these observations to a field guide for HCBs. Visual assessments require no special equipment and provide a rapid qualitative evaluation of the presence of planktonic or benthic cyanobacteria. With guidance materials and safety precautions, trained members of the public (citizen scientists and community monitors) can learn to recognize accumulations of cyanobacteria that may pose a health risk (see bloomWatch and the Lake Champlain Committee cyanobacteria monitors). Teaching the public to assess cyanobacteria presence is especially important because they can notice changes in water quality between monitoring events or evaluate small water bodies that are not routinely monitored by professional staff.
Visual assessments supported by guidance materials allow anyone recreating on or near the water to decide whether they, their children, or their pets should contact or drink the water. Photographs documenting conditions can be shared with local and state health officials, allowing officials to conduct further tests and notify the public. This approach also serves an important outreach and educational function.
Most states provide information on how to recognize cyanobacteria using visual cues and accept photographs as documentation for the presence of HCBs. Visual assessments in conjunction with targeted water quality monitoring and a communication platform can also be used to routinely share information about water conditions across a larger geographic area (see Vermont’s CyanoTracker Program and the New York State Harmful Algal Blooms Notifications Page).
Pros and cons. Visual assessments are quick and inexpensive. They require no specialized equipment, provide data immediately, and do not require extensive training or expertise. They can be an important decision-making tool when a potential HCB is encountered by the general public in areas that are not routinely monitored. Large numbers of sites can be assessed using this method with reasonable cost. Advances in machine learning and automated classification software may facilitate image processing. These assessments are most frequently used for planktonic cyanobacteria but may also be used for benthic HCBs. Visual assessments may be used to evaluate the presence or absence of cyanobacteria, but they cannot provide information on the presence of cyanotoxins.
Training materials and reference photographs improve the consistency and accuracy of assessments made by the general public and stress safety precautions. Reports from inexperienced individuals may require follow-up with the individual or a nearby partner organization. Your response plan should outline basic required information and the steps that will be taken to confirm HCBs reported by the general public.
|
Other guidance materials include:
|
4.3.1.2 Jar and Stick Tests
- Result: Qualitative
- Sampling type: Point sampling
- Turnaround time: <1 day
- Level of training: Novice
- Laboratory required: No
- Relative cost: $
- Cyanobacteria presence/absence: Suitable
- Cyanobacteria identification: Not suitable
- Cyanobacteria density: Not suitable
- Cyanotoxin presence/absence: Not suitable
The jar test uses the ability of some cyanobacteria to regulate buoyancy to separate them out from common aquatic biota. Water containing the suspected HCB is captured in a glass jar or other clear container and allowed to rest for 15 to 30 minutes. Cyanobacteria typically float to the surface, while other algae and sediment sink to the bottom (Figure 4-4). This test is not useful to check for benthic cyanobacteria or planktonic cyanobacteria such as Cylindrospermopsis that do not form surface scums.

Figure 4‑4. Using the jar test to assess the presence of planktonic cyanobacteria. Results show a well-mixed sample (A), settled material not likely to be cyanobacteria (B), and floating material likely to be cyanobacteria (C).
Source: Kansas Department of Health and Environment. Used with permission.
The stick test can be used to separate planktonic cyanobacteria from common filamentous green algae. Planktonic HCBs often have an appearance like spilled paint on the water and will coat a stick or other item when it is dipped into the bloom. Filamentous green algae, which is nonharmful, can form clouds just under the water surface or floating accumulations. They rarely coat the stick and will easily lift out of the water in long, hair-like strands (Figure 4-5). Benthic cyanobacteria may also lift easily out of the water but typically do not look like hair.
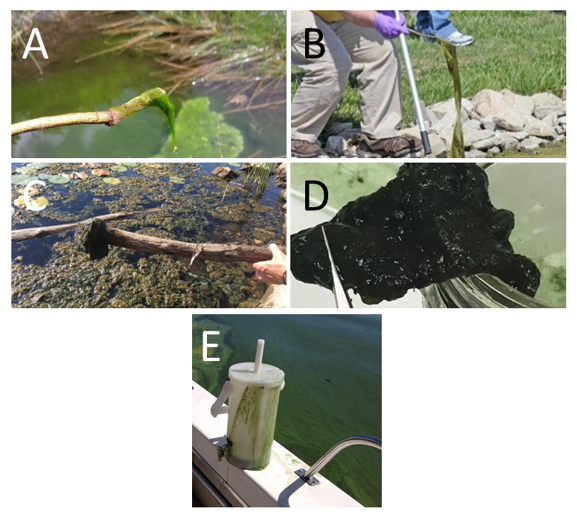
Figure 4‑5. Using the stick test to assess the presence of cyanobacteria. Photos A and B show filamentous green algae, which has a hair-like appearance. Some benthic cyanobacteria may be picked up with a stick (Photos C and D) but do not look like hair. Planktonic cyanobacteria blooms can coat surfaces like sticks and this churn splitter with a layer that looks like paint (Photo E).
Source: Amanda McQuaid, New Hampshire Department of Environmental Services (A); Kansas Department of Health and Environment (B); Lake Champlain Committee (C); Vermont Department of Environmental Conservation (D, E). Used with permission.
Pros and cons. Many states provide guidance on jar and stick tests (see examples from Minnesota and Kansas). Like the visual assessments, jar and stick tests require little equipment, and they can be carried out by anyone using a small amount of information (photographs or descriptions) to decide whether planktonic cyanobacteria might be present. Both tests can be used to support visual assessments. There is no cost associated with these tests, so an unlimited number of sites can be assessed with these methods. Training and reference photographs assist the general public in distinguishing planktonic cyanobacteria from similar phenomena like pollen. Benthic cyanobacteria are not easily recognized using the jar test and can be confusing when using the stick test. Neither test can identify cyanobacteria to genus or provide information on the presence of cyanotoxins.
4.3.1.3 Pigments
- Result: Quantitative
- Sampling type: Point sampling
- Turnaround time: <1 day to 3 days
- Level of training: Intermediate
- Laboratory required: Not always
- Relative cost: $$
- Cyanobacteria presence/absence: Suitable
- Cyanobacteria identification: Not suitable
- Cyanobacteria density: Suitable
- Cyanotoxin presence/absence: Not suitable
Photosynthetic organisms like cyanobacteria use a variety of pigments to capture the energy contained in sunlight. All algae use chlorophyll, the pigment with a typically green color. Cyanobacteria produce chlorophyll and two additional pigments—phycoerythrin and phycocyanin—that are not commonly produced by other freshwater organisms. By measuring pigments or the ratio of the pigments, we can estimate the abundance of cyanobacteria or algae that are present in the water.
The amount of photosynthetic pigment in algae and cyanobacteria cells is not constant. Cell health, cell age, and environment all influence the level of pigment present at any given time (Wetzel 2001). As sunlight passes into water, the amount of available light and the wavelengths of that light change. Algae and cyanobacteria adjust pigment ratios and quantities to enable efficient light harvesting under the conditions they are experiencing. As a result, pigment measurements should be supported with periodic microscopic or genetic evaluations to confirm taxonomy and corroborate biomass levels.
Real-time measurements. Photosynthetic pigments can be measured in real time using a variety of fluorometric sensors. Some can measure two photosynthetic pigments, typically chlorophyll and phycocyanin. Others measure only one, with phycocyanin being the most commonly used to evaluate the amount of cyanobacteria. New methods using pigments to quantify cyanobacteria and other photosynthetic organisms are in development as well. It is important to understand which pigment is measured by the sensor and the units used to quantify the data. For example, results can be provided as cells/mL or as relative fluorescence unit (RFU).
No sample preparation is necessary, and these instruments are frequently used to provide in situ estimation of cyanobacterial biomass – see NYDEC (2019) and Cyanos.org. These methods can also be used in high-frequency monitoring programs. Applications include buoys and other floating platforms, continuous monitoring of drinking water facility intakes, and beach surveillance (for example, The City of Toledo’s buoy monitoring system and USGS’ WaterQualityWatch for chlorophyll). Real-time measurements should be calibrated against laboratory measurements for better results and to assist with interpretation when compared with historical data.
Pros and cons for real-time measurements. Fluorometric sensors are easy to deploy and quickly provide quantitative information on cyanobacteria density. Because pigment concentration changes with cell health and environmental conditions, concentrations are highly variable. Development of local thresholds for pigments facilitates rapid evaluation of current condition and appropriate response level. Sensors range widely in sensitivity, ease of use, and cost. Periodic calibration is necessary for quality assurance and standardization if more than one unit is deployed. Turbid conditions can result in interference. In continuous deployments, care must be taken to account for biofilm buildup on probe surfaces. For more information on the use of real-time pigment measurements, see Marra (1997), Watras et al. (2017), and Bertone, Burford, and Hamilton (2018). For more information on quality assurance for continuous water quality monitoring, see USGS (2018).
Laboratory measurements. Photosynthetic pigments can also be extracted out of cellular material and evaluated in the laboratory. Typically, a volume of water is collected in the field, concentrated onto a glass fiber filter using vacuum, and then frozen. Freezing breaks down the cell wall and internal cellular material, facilitating pigment extraction by a chemical solvent. Pigment concentration in the solvent is determined by fluorometry or spectrometry.
Pros and cons for laboratory measurements. Laboratory analysis of chlorophyll a has historically been used to estimate total phytoplankton biomass. Many water bodies have long-term data that can be used to interpret current data. Laboratory data can be used to calibrate field probe accuracy. Some groups freeze water samples and evaluate them for phycocyanin content using field probes (Leland and Haney 2018). Because freezing and other laboratory extraction procedures liberate pigment from cellular material, laboratory results may return higher concentrations than field probe results. Laboratory analysis requires more time and expense but provides useful quality assurance data and greater data precision. Method development for laboratory analysis of phycocyanin is still a work in progress. This pigment is less commonly evaluated in the laboratory.
4.3.1.4 Remote Sensing
- Result: Quantitative and qualitative
- Sampling type: Indirect
- Turnaround time: <1 day to 1 day
- Level of training: Intermediate to expert
- Laboratory required: No
- Relative cost: $
- Cyanobacteria presence/absence: Suitable
- Cyanobacteria identification: Not suitable
- Cyanobacteria density: Suitable
- Cyanotoxin presence/absence: Not suitable
Water quality remote sensing platforms include satellite and airborne systems (manned and unmanned aircraft systems [UAS], drones), typically carrying imagers to measure reflected light energy (solar radiation) from the water surface and the upper part of the water column. Satellites typically provide broad spatial coverage at 10m–1km spatial resolutions on a consistent, repeated schedule, while airborne systems are capable of providing relatively higher spatial resolutions, though with more limited aerial coverage. As such, airborne systems offering relatively high spatial resolutions often provide the necessary spatial detail required to capture trends in small water bodies or along complex shorelines. Satellites do not provide useable data under cloud cover or cloud shadow and may be more prone to errors near shorelines due to mixed land and water signals. While airborne data may be collected under cloud cover, the data quality may still be influenced by cloud shadows. Many camera options for qualitative observations and narrow-band imaging sensors for quantitative measures have been adapted to drones and aircraft, including thermal capabilities. UAS have previously been demonstrated to be useful for HCB monitoring and even sample collection (Aguirre-Gómez et al. 2017). More detailed overviews on the use of drones for harmful algal bloom monitoring can be found in Kislik, Dronova, and Kelly (2018) and Wu et al. (2019).
Land missions and ocean color are the two general satellite categories most relevant to HCB monitoring. Land satellite missions and off-the-shelf cameras for airborne platforms typically use red, green, and blue spectral channels to generate true color images that can be used for qualitative assessment of visible changes. These images cannot accurately quantify concentrations of HCBs or relatively small changes in bloom characteristics. Ocean color missions and some land missions have more spectral bands and better signal-to-noise ratio required for quantifying changes. In this Section, we only mention satellite platforms that:
- are currently operational
- are suited for informing HCB management decisions
- have sufficient resolution to resolve lakes and some larger rivers
- are publicly available
- have a repeat measurement in the daily to weekly timeframe
One option is the Ocean and Land Colour Instrument (OLCI) on Sentinel-3 satellites (3A launched in 2016, and 3B launched in 2018). OLCI has a typical revisit time of 2–3 days with one satellite, but its spatial resolution (300-meter pixel size) limits observations to larger lakes and reservoirs. Another option is higher resolution land imagery (10–60-meter pixel size) from the Multi-Spectral Instrument on Sentinel-2 satellites (2A launched in 2015, and 2B launched in 2017) and Landsat series satellites. These imagers provide the best spatial resolution for smaller inland waters but are at a disadvantage when it comes to spectral resolution, signal-to-noise ratio, and, to some extent, temporal coverage. A more exhaustive review of satellite observations for water quality can be found in the International Ocean Colour Coordinating Group Report 17 (IOCCG 2018). At the time of writing, another IOCCG Report on the Observation of Harmful Algal Blooms with Ocean Colour Radiometers also has a section focused on cyanobacteria. In addition, Section 11 in the second edition of Toxic Cyanobacteria in Water (in press) will have information about using remote sensing to assist in planning monitoring programs.
What to do with remote sensing data. Remote sensing data can be used for near-real-time monitoring, as well as for historical analysis.Near-real-time data are available after the latency time, defined as the time it takes for data to be acquired, transferred, stored, and then accessed. Typical latency is less than 12–24 hours for most missions, but it can be as low as 3 hours for Sentinel-3 satellites and 1–5 hours for Landsat satellites. This does not include the time that may be required to process the image to a derived geophysical measurement. Satellite resolutions of 1 kilometer to 300 meters are most beneficial in large lakes, reservoirs, and estuaries; however, repurposed land satellites such as Landsat and Sentinel-2 have been proven effective in rivers and smaller systems with 10–30-meter resolutions.
Pros and cons. Remote sensing technologies, such as satellites, can provide a comprehensive view of a water body. These approaches help in tracking surface blooms and identifying changes in surface distributions over time. Typically, ocean color satellites only provide measures for larger estuary, lake, and river systems. However, land satellites can provide measures for smaller estuaries, lakes, and even rivers. For example, 300-meter Sentinel-3 data provide measures for approximately 1,800–15,000 U.S. lakes and 197 U.S. estuaries. Thirty-meter Landsat and Sentinel-2 data provide measures for approximately 170,000–276,000 U.S. lakes and reservoirs and 301 estuaries (see Clark et al. 2017, Schaeffer and Myer 2020 for more details). Availability of pigment sensors varies among satellites. Remote sensing does not give a clear understanding of what is happening in the lake, below the water’s surface. While these methods can be used to detect algal and cyanobacterial pigments for estimates of biomass, they cannot directly detect cyanotoxins.
4.3.1.5 Microscopy
- Result: Qualitative/quantitative
- Sampling type: Point sampling
- Turnaround time: <1 day
- Level of training: Intermediate
- Laboratory required: Yes
- Relative cost: $$
- Cyanobacteria presence/absence: Suitable
- Cyanobacteria identification: Suitable
- Cyanobacteria density: Suitable
- Cyanotoxin presence/absence: Not suitable
Microscopy is used to identify cyanobacteria and to quantify their abundance. Counting cells in a water sample under a microscope has traditionally been considered the “gold standard” for monitoring cyanobacteria and can assist in identifying a potentially toxic bloom. It is possible to identify many cyanobacteria to the genus or even species level if they have distinct morphological features (Figure 4-6). Cyanotoxins, however, cannot be detected using microscopy.

Figure 4‑6. Using a microscope to identify cyanobacteria.
Source: New Jersey Department of Environmental Protection. Used with permission.
Some training is required for taxonomic identification of HCBs. Several free taxonomic resources are available online, such as:
- ITRC’s Visual Guide to Common Harmful Cyanobacteria, included as part of this document
- Phyco Key from the University of New Hampshire
- Western Washington University’s Cyanobacteria Key
- Cyanos.org’s cyanoScope
- The National University of Ireland’s AlgaeBase
Many published taxonomic references are also available. Automated options for taxonomic identification of HCBs are becoming more available—see Section 4.3.1.7. Cyanobacteria taxonomy is constantly evolving, and names in older references have often changed. Some species of Anabaena are now classified as Dolichospermum, for example. Taxonomists should consult current literature and other sources to determine appropriate names for organisms they have identified. We have provided a crosswalk table in the Visual Guide Common Harmful Cyanobacteria.
Samples collected as part of routine monitoring programs are typically preserved at the time of collection; however, most taxonomic keys are based on characteristics of live specimens, and when working with samples of material that may contain unfamiliar taxa, most experienced taxonomists prefer live, unpreserved material. If you do not preserve the sample, microscopic analyses should be completed as quickly as possible after collection. Unpreserved samples degrade within a few days, especially if the material was collected as the HCB was breaking up.
Some important physical characteristics such as color or the presence of gas vacuoles (called aerotopes) may not be apparent after a sample has been preserved. Some preservatives may cause distortion of fragile cells or disintegration of colonies (Muller 2005). If you also plan to use preserved samples for molecular analyses such as polymerase chain reaction (PCR), be sure that the preservative is appropriate for the method. Details of microscopic methods and sample preparation can be found in the literature and other references (see Chorus and Bartram 1999, Meriluoto, Spoof, and Codd 2016).
Pros and cons. Microscopic methods to identify and enumerate cyanobacteria can be inexpensive and use minimal equipment: a microscope, slides or a counting chamber, and a pipette (see Cyanos.org/cyanoScope). Basic identification can be conducted by community scientists with basic training. State laboratories, universities, and commercial facilities usually have higher grades of equipment and more extensive training. Microscopic methods are also consistent with regulatory guidance (USEPA 2019e). Precise taxonomic identification can be time-consuming and must be performed by trained technicians. Because only a small portion of the sample is analyzed, only cells of abundant species may be counted accurately; rare species are often missed. In addition, preservatives may disintegrate or distort fragile species, resulting in underestimated cell counts.
Several sources of variability and error should be considered when using microscopy. The small volume examined under the microscope may not be representative of the water body overall, since cyanobacteria distribution is often patchy. Counting dense samples, filamentous, or colony-forming species can be difficult and subject to substantial error. This variability may be minimized by collecting large samples and mixing them well or by evaluating a larger number of samples collected over time or space.
4.3.1.6 Genetic Methods for Identification
- Result: Quantitative
- Sampling type: Point sampling
- Turnaround time: <1 day
- Level of training: Intermediate
- Laboratory required: Yes
- Relative cost: $$
- Cyanobacteria presence/absence: Suitable
- Cyanobacteria identification: Suitable
- Cyanobacteria density: Potentially suitable
- Cyanotoxin presence/absence: Not suitable
Gene target analyses. Genetic methods create many exact copies of (amplify) a particular sequence of molecules (nucleotides) in the sample DNA. In this way, they allow us to see whether the genes of target organisms are present in the sample, particularly those that may be rare in the original sample. For example, genetic methods can be used to evaluate the amount of total cyanobacteria, specific genera of cyanobacteria, or gene clusters associated with cyanotoxin production. See Section 4.3.2.6 to learn more about genetic methods for cyanotoxins.
Polymerase chain reaction (PCR), quantitative real-time PCR (qPCR), reverse transcription qPCR (RT-qPCR), and digital PCR (dPCR) are all types of genetic analysis methods. PCR provides information only about the presence or absence of target DNA in a sample. qPCR and dPCR can be used to quantify the concentration of the genes of a target organism in a sample (Coyne et al. 2005, Medlin and Orozco 2017). Multiplexing offers a way to efficiently amplify several gene targets simultaneously (Ngwa, Madramootoo, and Jabaji 2014, Al-Tebrineh et al. 2012).
The steps in performing genetic assays typically include collecting a water sample in the field; separating suspended matter (including cyanobacteria) by centrifugation or filtration, cell lysis, and extraction of genetic material from the pellet or filter; adding enzymes, primers, deoxynucleotide triphosphates, and a buffer; and, finally, amplifying DNA and detecting a specific target sequence in an instrument called a thermal cycler. Samples are usually sent to a laboratory for analysis, although recent advances in instrumentation allow for field measurements.
Community analyses. Measuring the concentrations of genus- or species-specific gene targets may be useful in settings where a particular organism or a limited number of organisms are expected to bloom on a recurring basis; however, to monitor for many different organisms at once, including in situations where the identity of the cyanobacteria is not known ahead of time, community analyses may be a more efficient method. To date, these methods have been used primarily for academic research, but as the costs of these analyses rapidly decrease, they may be used more widely for monitoring.
One type of community analysis is 16S rRNA gene sequencing. With this method, PCR is used to target and amplify portions of the bacterial 16S rRNA gene, a gene that has shared portions among bacteria but that also varies enough among bacteria to distinguish different genera or species. Amplified sequences are compared to a 16S reference database to identify organism types. The overall community structure can then be characterized. This method has been used to look at cyanobacterial community composition during different blooming stages (Lu et al. 2020).
Another type of community analysis is shotgun metagenomic sequencing. In contrast to 16S sequencing, shotgun sequencing targets not only 16S rRNA genes but also all available genomic DNA in a sample. Because shotgun sequencing includes more genetic information, the sequences can be used to look at characteristics of the bacteria other than identity, such as ability to produce toxins (Otten et al. 2016) or to fix nitrogen gas or scavenge phosphorus (Lu et al. 2019). Similarly, metatranscriptomic sequencing can be used to examine community-wide gene expression (Harke and Gobler 2013).
Pros and cons. Genetic methods offer efficient and powerful alternatives to microscopic methods for detection and enumeration of cyanobacteria (Sellner, Doucette, and Kirkpatrick 2003). They can be used to detect cyanobacteria species that cannot be reliably identified by microscopy due to their small size or lack of distinguishing features, or to identify fragile species that disintegrate or distort in the presence of chemical fixatives (Doll et al. 2014, Godhe, Anderson, and Rehnstam-Holm 2002). Genetic methods have low detection limits compared to microscopic methods, which can help give early warning of blooms (Te, Chen, and Gin 2015). Assays are rapid and efficient, such that tens of samples can be collected, extracted, and analyzed in a single day.
Genetic methods return a quantitative result, typically expressed as the number of gene copies per unit. This is not easily translated into the more typical estimates used for cyanobacteria (cells/mL); however, genetic methods compare favorably with more traditional methods and can be useful proxies to evaluate HCBs.
Current genetic methods typically require laboratory analysis, although handheld field instruments have been developed and are being used more and more. Equipment is expensive and operating the equipment and analyzing the results requires experience and expertise. In addition, environmental samples may contain inhibiting compounds that result in underestimated amounts of target DNA present. The potential for cross-contamination is high, so care must be taken to clean field equipment between locations. Check with the laboratory for its recommendation on appropriate procedures. Problems with inhibitors can be overcome by diluting samples or by using dPCR (Te, Chen, and Gin 2015).
Specific primers are not available for all cyanobacteria species, which may make identification of unusual species by targeted gene analysis difficult; community analyses may be necessary to identify all species present. Finally, it can be difficult to compare historical monitoring data with results from genetic analyses.
4.3.1.7 Semi-automated Classification and Machine Learning
- Result: Quantitative
- Sampling type: Point sampling
- Turnaround time: <1 day
- Level of training: Intermediate
- Laboratory required: Yes
- Relative cost: $$
- Cyanobacteria presence/absence: Suitable
- Cyanobacteria identification: Suitable
- Cyanobacteria density: Suitable
- Cyanotoxin presence/absence: Not suitable
Semi-automatic classification and machine learning identify and enumerate cyanobacteria and other plankton by first photographing individual cells as a sample flows through an imaging flow cytometer (Figure 4-7). The images are then processed by machine learning software that identifies cyanobacteria based on cell morphology—cell shape and size—and provides density estimates based on statistical groupings of shared morphologies (Qu et al. 2019). To distinguish cyanobacterial cells from other cells, cytometers may be coupled with fluorometric probes that identify cyanobacteria based on the unique spectral signature of their pigments. These systems are being used in both freshwater and marine environments (Benfield et al. 2007).
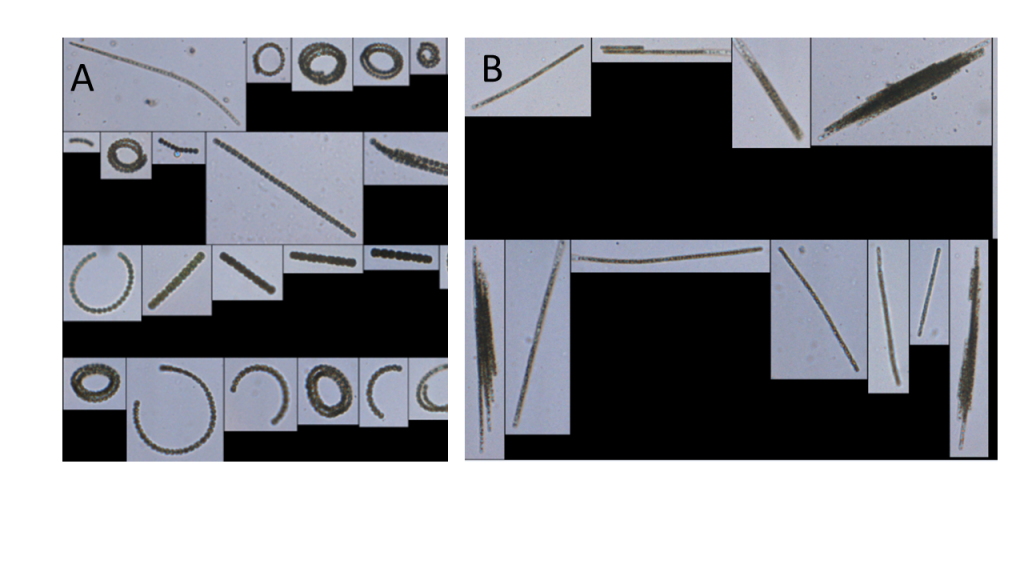
Figure 4‑7. Photos captured by an imaging flow cytometer: Dolichospermum (A) and Aphanizomenon (B).
Source: Hunter Adams, City of Wichita Falls, TX. Used with permission.
Pros and cons. Since semi-automatic classification and machine learning do not require a taxonomist to manually evaluate cyanobacteria samples like traditional microscopy, this method can provide greater accuracy, faster turnaround times, and lower cost (Deglint et al. 2018). However, taxonomic skills are necessary to confirm machine identifications and build the “library” as new species are found. It can be difficult to compare data from historical microscopic analysis to those from automated systems (Hrycik, Shambaugh, and Stockwell 2019).
Recent advances in machine learning technology may soon allow for cyanobacteria identification and enumeration in your own water quality laboratory by processing images that are captured under traditional microscopes (Jin et al. 2018). Furthermore, applications for smartphones or fixed cameras in the field, are being tested for identifying cyanobacterial blooms in real time.
4.3.2 Toxin Testing
Cyanotoxin monitoring is an integral part of any cyanobacterial bloom response plan. HCBs sometimes produce toxic compounds known as cyanotoxins. These present a danger to human and animal health and can persist after the bloom has disappeared. Not all cyanobacteria are able to produce toxins, and those that can produce toxins do not always do so. A bloom can begin to produce toxins at any time, and the factors that drive toxin production are not well defined. There is no way to visually distinguish a toxic bloom from a nontoxic bloom. It is important to remember that a cyanotoxin sample can tell you only about conditions at the time the sample was collected – it cannot predict conditions in the future. Please refer to Section 3 and Section 2 of HCB-2 for an overview of cyanotoxins and harmful compounds that may be produced by cyanobacteria.
Most cyanotoxins remain in the cells unless the cell wall is damaged or the HCB has begun to break down. This is true for the cyanotoxins anatoxin and microcystin; however, cells producing cylindrospermopsin may release toxins from live cells that are neither damaged or dying (USEPA 2014). As a result, concentrations of cyanotoxins in the water (extracellular) and cyanotoxins in the cells (intracellular) can be very different. It is important to consider this distinction when deciding how and where to sample for cyanotoxins. Most laboratory analyses include a step to break open the cell wall and release intracellular toxins to provide a measure of the total amount of cyanotoxin present. This may not be the case for the quicker field methods. It is important to understand which methods have been used when comparing your results to other lakes.
Cyanotoxins are not one compound, but instead represent a large group of highly variable compounds with different toxicology. They have both short- and long-term exposure risks, so the frequency of monitoring depends on the typical expected exposure route—for example, drinking water, recreational contact with water, or fish consumption. Extraction of cyanotoxins from plant, animal, and human tissue requires careful preparation, and a single extraction method may not be suitable for all types of tissue (Sanan et al. 2019). In addition, some methods are subject to interference from other environmental compounds or contaminants. Be sure that you understand the appropriate way to collect and store a sample for the cyanotoxin method you have chosen.
Because cyanotoxins are highly variable compounds, many toxin analysis methods are only capable of evaluating only one kind of toxin. An enzyme-linked immunosorbent assay (ELISA), for example, will provide information about anatoxin only, while mass spectrometry can provide concentrations for several different cyanotoxins and their variants in a single analysis. No single analytical method can provide information about all possible cyanotoxins that may be present in a water body, and analytical standards and standard reference materials are not available for all cyanotoxins. Most HCB monitoring programs focus on one or two cyanotoxins only. This is due partly to the cost of running many analyses, but also because cyanotoxin guidance values, which provide an estimate of when concentrations may be high enough to be harmful, have not been developed for all cyanotoxins. See Section 3 for more information.
Table 4-2 provides an overview of analytical methods suitable to evaluate cyanotoxins. For a more complete description of each method, use the hyperlink to access its Section in our document. Our interactive tool can also be used to explore methods. Methods may not meet your state’s regulatory and health requirements, so be sure to check before deciding which method you will use. Many jurisdictions require additional testing to confirm results from rapid field test methods.
Table 4‑2. Monitoring methods used to identify and measure cyanotoxins
| Methods for Identification and Enumeration | Result Type | Turnaround Time |
| Strip Tests/Dipsticks | Semi-quantitative | <1 day |
| Protein Phosphatase Inhibition Assay (PPIA) | Quantitative | <1 day |
| ELISA | Quantitative | <1 day |
| Mass Spectrometry | Quantitative | 1–3 days |
| Chromatography | Quantitative | 1–3 days |
| Genetic Analysis for Cyanotoxins | Qualitative | 1–3 days |
4.3.2.1 Strip Tests/Dipsticks
- Result: Semi-quantitative
- Sampling type: Point sampling
- Turnaround time: <1 day
- Level of training: Novice
- Laboratory required: No
- Relative cost: $$
- Cyanobacteria presence/absence: Not suitable
- Cyanobacteria identification: Not suitable
- Cyanotoxin presence/absence: Suitable
- Congener-specific cyanotoxin concentrations: Not suitable
- Total cyanotoxin concentrations by class: Suitable
The ability to rapidly screen and analyze a water body for the presence of cyanotoxins is important for decision making. While there are no visual indicators of cyanotoxin presence in an HCB, commercial vendors have developed a way to rapidly (<1 hour) screen and evaluate the presence of cyanotoxins using dipstick technology. After an optional cell lysis step to release intracellular cyanotoxins, an antibody/antigen reaction is used to evaluate the presence of cyanotoxins in the sample.
Pros and cons. While the testing and results are rapid, sample preparation and assay interpretation are influenced by the experience of the sampler and the quality and condition of the water sample (LeDuc, Christensen, and Maki 2020). Possible interference from unknown contaminants can cause assay failure, and strips can be difficult to interpret. Many states require that results from dipsticks and test strips be confirmed with a quantitative test such as mass spectrometry. Examples of interpreting dipstick results can be seen in the Harmful Algal Bloom (HAB) Guidance Document for Montana (MDEQ, MDPHS, and MFWP 2018) and the Oregon Harmful Algae Bloom Surveillance (HABS) Program Recreational Use Public Health Advisory Guidelines for Cyanobacterial Blooms in Freshwater Bodies (OHA 2019). Currently, dipsticks and strip tests are available only for microcystin, anatoxin-a, and cylindrospermopsin.
4.3.2.2 Protein Phosphatase Inhibition Assay (PPIA)
- Result: Quantitative
- Sampling type: Variable
- Turnaround time: <1 day
- Level of training: Intermediate
- Laboratory required: Yes
- Relative cost: $$
- Cyanobacteria presence/absence: Not suitable
- Cyanobacteria identification: Not suitable
- Cyanotoxin presence/absence: Suitable
- Congener-specific cyanotoxin concentrations: Not suitable
- Total cyanotoxin concentrations by class: Suitable
PPIAs are used to test for microcystin and nodularins. These cyanotoxins strongly affect the function of protein phosphatase enzymes, and the level of inhibition can be measured using colorimetry. Protein phosphatase activity produces paranitrophenol, which has a yellow color at higher pH. When microcystin and nodularin bind to protein phosphatases, the enzymes can no longer produce paranitrophenol, decreasing the amount of yellow coloring. PPIA therefore directly measures the biological effect of these cyanotoxins, which is then correlated to microcystin and nodularin concentration (Carmichael and An 1999). In contrast, other methods like ELISA and mass spectrometry rely on structural components of the cyanotoxin molecule for quantitation.
Pros and cons. Ease of use and sensitivity of this assay are similar to ELISA. Because PPIA measures a biological effect, it cannot distinguish between microcystins and nodularins. Typically, a second test is used to identify which cyanotoxin is present; however, CIPPIA, a modified PPIA incorporating polyclonal antibodies, has been shown to successfully distinguish among several microcystin variants and nodularin (Metcalf, Bell, and Codd 2001). False positives can occur if other types of protein phosphatases are active in the sample, but this is minimized by good sample preparation.
4.3.2.3 Enzyme Linked Immunosorbent Assay (ELISA)
- Result: Quantitative
- Sampling type: Variable
- Turnaround time: <1 day
- Level of training: Intermediate
- Laboratory required: Yes
- Relative cost: $$
- Cyanobacteria presence/absence: Not suitable
- Cyanobacteria identification: Not suitable
- Cyanotoxin presence/absence: Suitable
- Congener-specific cyanotoxin concentrations: Not suitable
- Total cyanotoxin concentrations by class: Suitable
ELISA is a type of biological assay that uses reactive proteins known as antibodies to detect and quantify cyanotoxins. While ELISA can detect individual groups of cyanotoxins (for example, microcystin or cylindrospermopsin), it does not distinguish between the different molecular structures (known as congeners) of those groups, such as microcystin-LR or microcystin-RR. Some congeners may have higher levels of toxicity than others, and during a bloom, congener composition can change (Monchamp et al. 2014). To reduce the variability and speed testing, ELISA detects and quantifies total cyanotoxin abundance in a sample.
Commercial ELISA kits for cyanotoxins work on an indirect or direct competitive principle. Cyanotoxin binding proteins are placed on an absorbent plastic plate. After processing, a sample that is suspected of containing cyanotoxins is placed onto that plate as well. Cyanotoxin-specific antibodies are then added and allowed to bind to the toxin or to the protein already on the plate. A colorimetric response indicates the amount of cyanotoxin present. By comparing the color levels to standards of known concentrations that are run with each assay, it is possible to quantify the level of cyanotoxin in the sample.
Pros and cons. ELISA provides quantitative information on specific cyanotoxins relatively quickly compared to other quantitative methods. Correlation of ELISA results with other methods varies with cyanotoxin (Gaget et al. 2017). ELISA does not provide information on congener types and, depending on the test kit, may not capture all of the targeted cyanotoxin. Results may be subject to cross-reactivity (non-toxin compounds erroneously identified as cyanotoxins), so it is very important to understand the method’s limitations, any cross-reactivity, and sample preparation requirements. In addition, some jurisdictions may require that ELISA results be confirmed using a second analytical method such as liquid chromatography–mass spectrometry (LC-MS).
4.3.2.4 Mass Spectrometry
- Result: Quantitative
- Sampling type: Variable
- Turnaround time: 1–3 days
- Level of training: Expert
- Laboratory required: Yes
- Relative cost: $$$
- Cyanobacteria presence/absence: Not suitable
- Cyanobacteria identification: Not suitable
- Cyanotoxin presence/absence: Suitable
- Congener-specific cyanotoxin concentrations: Suitable
- Total cyanotoxin concentrations by class: Not suitable
Mass spectrometry is a powerful chemistry technique that analyzes samples to determine which compounds might be present by comparing the sample to known analytical standards. Mass spectrometry initially ionizes the compounds present in a water sample and, by comparing the mass to the net charge ratio (m/z), sorts and plots the cyanotoxins by molecular size and relative abundance.
Cyanotoxins can have many different congeners, and this technique can identify all congeners present. It can also detect much lower concentrations than any of the other cyanotoxin analysis methods. Depending on how this method is used, it can take substantially more time to prepare and analyze samples; however, it can provide much higher resolution and insight into the full cyanotoxin profile of an HCB event.
Pros and cons. Mass spectrometry is a highly specific and precise method used for the identification and quantification of all cyanotoxins; however, it is costly, and its turnaround time is longer than for most other methods. This method is often used for a secondary confirmation for results obtained using other, less expensive methods.
4.3.2.5 Chromatography
- Result: Quantitative
- Sampling type: Variable
- Turnaround time: 1–3 days
- Level of training: Expert
- Laboratory required: Yes
- Relative cost: $$$
- Cyanobacteria presence/absence: Not suitable
- Cyanobacteria identification: Not suitable
- Cyanotoxin presence/absence: Suitable
- Congener-specific cyanotoxin concentrations: Suitable
- Total cyanotoxin concentrations by class: Not suitable
Chromatography refers to the technique of separating a mixture by passing it through a phase shift (gas chromatography) or by dissolving a sample in a solvent (liquid chromatography). The resulting compounds are separated by passing through selective media and then compared to known standards. Chromatography is often combined with other chemistry techniques to help detect and quantify cyanotoxins. High-performance liquid chromatography (HPLC), liquid chromatography-mass spectrometry (LC-MS), and liquid chromatography–triple quadrupole mass spectrometry (LC-MS/MS) are among the most commonly used chromatography methods to analyze cyanotoxins. Each chromatography method operates at a certain speed and has a certain detection specificity.
Generally, HPLC works by separating cyanotoxins based on solubility by pushing samples through a column under high pressure. HPLC combines a detector and emitter, usually of UV light, and measures samples as they move through the gradient. LC-MS works by ionizing the samples first and combining the physical chromatography nature of HPLC and the mass particle separation of mass spectrometry. LC-MS can simultaneously separate and distinguish many different classes of cyanotoxins in a mixture, though there is some overlap. Despite this, LC-MS is a sensitive method that can detect down to the nanogram/liter level. LC-MS/MS has a higher selectivity and better resolution than traditional LC-MS. This is due to the additional mass spectrometry detector, which detects and selectively filters out ions of interest. This additional filtering step, combined with fragmentation of the ions, produces a higher precision image of the molecular patterns present in the sample.
Pros and cons. Analysis by chromatography requires the use of trained staff using specialized equipment, meaning both an increase in cost and turnaround time when compared to other methods. Chromatography is generally able to quantify to levels much lower than the other methods listed, and it is often used with other analytical techniques, primarily mass spectrometry. Samples that are processed for chromatography are often sent to independent laboratories and may require different sample collection and storage conditions than for other analytical techniques. Chromatography is most often used to confirm results of other detection methods and to identify emerging or novel cyanotoxins that an HCB could be producing that are not detectable by other methods.
4.3.2.6 Genetic Analysis for Cyanotoxins
- Result: Quantitative
- Sampling type: Variable
- Turnaround time: 1–3 days
- Level of training: Expert
- Laboratory required: Yes
- Relative cost: $$$
- Cyanobacteria presence/absence: Not suitable
- Cyanobacteria identification: Not suitable
- Cyanotoxin presence/absence: Suitable
- Congener-specific cyanotoxin concentrations: Suitable
- Total cyanotoxin concentrations by class: Not suitable
Similar to the use of molecular genomics for cyanobacterial taxonomy, these methods evaluate potential toxicity by targeting the genes that encode the cyanotoxin (Chiu et al. 2017). The presence of toxin-producing genes indicates that organisms are capable of generating toxins, but it does not confirm that organisms are actually producing toxins; however, studies have shown that the presence of cyanotoxin genes indicates that cyanotoxins are present to some degree in the water (Christensen et al. 2019). Additional methods have been developed to look not only for the presence of toxin-producing genes but also for the expression of these genes. This approach has been used to detect the expression of microcystin-producing genes from lake water samples during Microcystis blooms (Lu et al. 2020, Pimentel and Giani 2014, Wood, Holland, and MacKenzie 2011). Water body-specific relationships between the presence of genes and cyanotoxin concentrations may be identified using this approach.
Examples of genomic studies for toxins include:
- Multiplex qPCR assays have been developed targeting microcystin synthase genes (mcy) in Anabaena, Planktothrix, and Microcystis spp. Using this assay, Ngwa, Madramootoo, and Jabaji (2014) showed that toxic strains of Microcystis dominated the population in Missisquoi Bay, Canada, and were present even when this species was not detected by microscopy.
- Baker et al. (2013) described a semi-automated multiplex-tandem qPCR assay for sensitive detection of biosynthetic genes for microcystins, nodularins, cylindrospermopsins, and saxitoxins, with a diagnostic specificity of 100% for each assay.
- Water quality monitoring by the Big Valley Band of Pomo Indians on Clear Lake uses qPCR monitoring for toxin genes as a cost-effective indicator.
- Pimentel and Giani (2013) also used a multiplex approach to determine that the fraction of toxic cyanobacteria in a Brazilian reservoir decreased as cell numbers increased but were the major fraction (up to 100%) of the population at low cell densities.
- Zuo et al. (2018) found that the ratio of mcyD to mcyA is usually a good predictor of toxicity—mcyB is usually universally present in blooms dominated by Microcystis (toxic and nontoxic).
- The Ohio Environmental Protection Agency’s HAB response strategy for drinking waters calls for qPCR assessment for total cyanobacteria and toxin-producing genes (mcyE gene for microcystin, cyrA gene for cylindrospermopsin, and sxtA gene for saxitoxin). Based on these screening data, additional samples may be taken and analyzed for toxins (Ohio EPA 2020).
- McKindles et al. (2019) completed a multiplex analyses of cyanobacteria in Lake Winnipeg and detected the presence and expression of several cyanotoxin genes, as well as the co-occurrence of cyanotoxin genes at low levels in the phytoplankton population.
Pros and cons. qPCR for cyanotoxin genes can be a cost-effective approach to fully characterize the cyanotoxins that may be present in a water body over time; however, qPCR cannot measure cyanotoxin concentration directly. Genetic methods can differentiate potentially toxic cyanobacteria from nontoxic cyanobacteria, such as different strains within the Microcystis genus (Pearson and Neilan 2008), something microscopic methods cannot. These genes have been shown to be a better indicator of the potential of an HCB to produce cyanotoxins than measures of total cyanobacterial biomass (USEPA 2019e). Environmental samples may contain inhibiting compounds that result in underestimated amounts of target DNA present. Potential for cross-contamination is high, and careful sampling protocols are required. Specific primers are not available for all cyanotoxins, which may make identification of unusual ones difficult. It also can be difficult to compare historical monitoring data with results from genetic analyses.
4.4 Selecting Appropriate Sample Collection Methods for Your Lake’s HCB Event
The methods that you use to monitor HCBs and the location where samples are collected are important aspects of your monitoring program. You might need multiple approaches or a tiered approach to achieve your program’s objectives. Information found elsewhere in this document will help you design your monitoring program to meet the goals of your HCB response plan (Section 5) and your HCB management plan (Section 3.4). Guidance on developing monitoring programs is also available for planktonic blooms (Graham et al. 2008, Newcombe et al. 2010, USEPA 2019e, USGS 2018) and benthic communities (Biggs and Kilroy 2000, Gaget et al. 2020).
Figure 4-8 presents monitoring methods that can be considered when sampling HCBs. These are grouped by water body type and location. Because HCBs and circumstances vary, lake managers will need to decide which location in the water body is the most representative and which collection type best meets the monitoring objective. For instance, if the wind has confined a floating HCB to a swimming beach at a lake, managers could use several approaches—a grab sample to characterize a specific area of the swimming beach at the downwind shoreline where HCBs often concentrate, a composite sample collected along the shoreline to characterize the swimming beach as a whole, or a series of separate samples along the beach to assess variability.
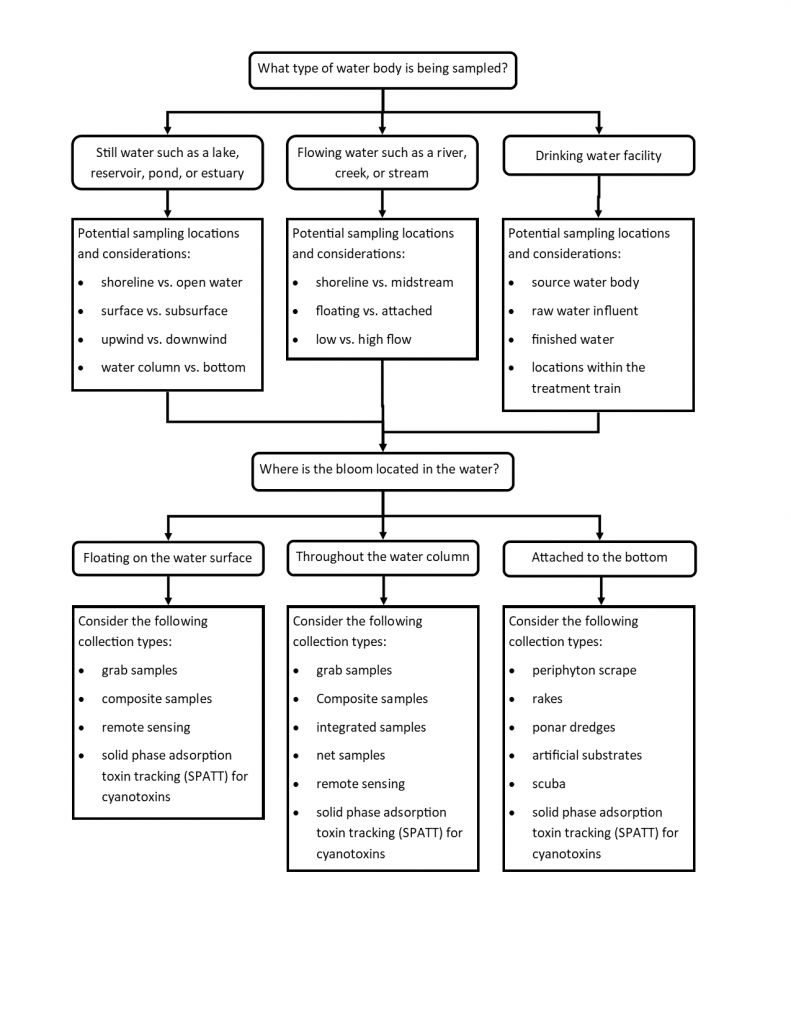
Figure 4‑8. Considerations in selecting HCB monitoring approaches.
Source: ITRC. Used with permission.
4.4.1 Planktonic HCB Sampling Methods
People are most familiar with the surface scums and highly discolored water that is present during HCBs. These indicators are caused by planktonic cyanobacteria species that float in the water. Planktonic HCBs may also be distributed throughout the water column at lower concentrations that are less easily seen. Some cyanobacteria can regulate their buoyancy so precisely that they form a layer well below the surface that cannot be seen at all. Several methods may be used to collect samples from planktonic HCBs, depending on the final use of the data.
4.4.1.1 Grab and Composite Samples
A grab sample obtained by dipping a wide-mouth container into the water can often provide the necessary biomass needed for taxonomic identification and cyanotoxin testing. Grab samples can also be collected from surface scums. Grab samples are often used to capture the “worst case” example of the HCB. A trained person using microscopic examination can identify cyanobacteria in grab samples to a taxonomic level that is sufficient to indicate which potential cyanotoxins might be present. This screening-level data may be sufficient for drinking water suppliers or public health agencies to take action under their management plans, such as starting treatment for cyanobacteria or cyanotoxins, using another water source, or closing a beach. Grab samples can also be used for quantitative cell counts, pigment analysis, toxicity tests, and genetic analyses.
Some states and other entities use another type of grab sample to collect a sample that may be more representative of the upper water column in the nearshore area. A sampler wades into the water to a depth of 1 meter or reaches into the water from an elevated location like a dock. The sampler then plunges an uncapped, inverted bottle into the water a full arm’s length or to an assigned depth, typically between 0.25 meter and 0.5 meter. Using a sweeping motion, the sampler turns the bottle upright, brings it back to the surface, and caps it. This approach can be used at beaches, boat ramps, and other nearshore areas (Figure 4-9).

Figure 4‑9. Examples of several ways to collect grab samples for cyanobacteria.
Source: New Jersey Department of Environmental Protection. Used with permission.
Since cyanobacteria populations are found in patches, multiple grab samples might be needed to understand the spatial coverage of HCBs. A composite sample composed of three or more grab samples could be used to characterize HCBs across a wider area—a beach, for example. Equal amounts of grab samples from locations across the beach are poured into a single container, mixed well, and evaluated using the methods described earlier in this Section.
To fully quantify data obtained from grab samples (for example, as cells/mL or µg of microcystin/L), a known volume of water must be collected. If the volume is not known, results are presented as relative values (present/absent or percent of total cells counted).
4.4.1.2 Integrated Samples
Integrated samples capture all water and the plankton in it to a specific depth. They are typically collected in the epilimnion, the upper layer of a stratified water body, where the sunlight penetrates and where planktonic organisms are most abundant. A typical depth is 2–3 meters, collected in the deepest segment of the water body from a boat. Cyanobacteria may be dispersed throughout the entire epilimnion. Integrated sampling provides a way to characterize the average planktonic population. Integrated samples can be used for cyanobacteria identification, cell counts, pigments, cyanotoxins, and genetic analyses. As for grab samples, you must collect a known volume of water to fully quantify your results. Grab samples represent average values for the depth sampled (for example, cells/mL in the epilimnion).
To collect a whole water plankton sample, you need a plastic tube of known diameter and length. This can be a rigid plastic PVC tube or a flexible hose, weighted at the bottom and with a string attached near the bottom. Tie the upper end of the string to the boat until needed. Lower the lower opening of the sampling tube vertically from the boat until you reach the desired depth. Then, cork or otherwise cap the upper opening to create a vacuum. Raise the lower end of the tube or hose out of the water (or pull it up using the string). Empty the tube into a large container by releasing the suction. USEPA’s QAPP for the Cyanobacteria Monitoring Collaborative Program (2017b) outlines how to sample using an integrated tube sampler.
4.4.1.3 Plankton Nets
You can also use a plankton net to collect an integrated sample; however, this method will not retain all organisms. Nets are constructed of porous mesh, which allows organisms smaller than the mesh openings to pass through the net while larger organisms are captured. The advantage of using a plankton net is that it concentrates phytoplankton from a large volume of water as it is pulled horizontally or vertically through the water. This is very useful during pre-bloom conditions, where cyanobacteria are less abundant. Nets can be difficult to use during HCBs, however, because they may clog.
Net samples can be used for cyanobacteria identification, cell counts, pigments, cyanotoxins, and genetic analyses. To quantify net plankton results, you must know the mesh size, the diameter of the net opening, and the length or depth of water sampled. Vermont’s Champlain Cyanobacteria Monitoring QAPP/Workplan outlines how to sample using a plankton net. Because plankton nets do not capture all the cyanobacteria that may be present (and may underestimate cyanotoxin concentration as a result), it is important to consider how you will use the resulting data. For more information, see (Graham and Jones 2007) and Lim-Tex’s Fundamentals of CyanoCasting handbook.
4.4.2 Sampling Benthic or Attached Cyanobacteria
Planktonic sampling methods cannot be used effectively on benthic cyanobacteria. Benthic cyanobacteria are typically filamentous and form mats on hard substrates like rocks and soft substrates, including sediment or sand (Figure 4-10). Due to their tendency to grow in dense tangles, benthic mats of cyanobacteria can sometimes appear like blue-green rugs. These mats, also called biofilms, may include filamentous green algae, diatoms, and other algae, as well as bacteria, polysaccharides, and fungal hyphae. Mats washed up on shore can dry out to become “chips” that are attractive to dogs and may contain cyanotoxins (Backer et al. 2013). Benthic HCBs are known to produce many of the same toxins as the planktonic forms (see Table 3-1). Although many of these mats may be toxic, isolating the cyanobacteria-specific toxin-producing species is difficult.
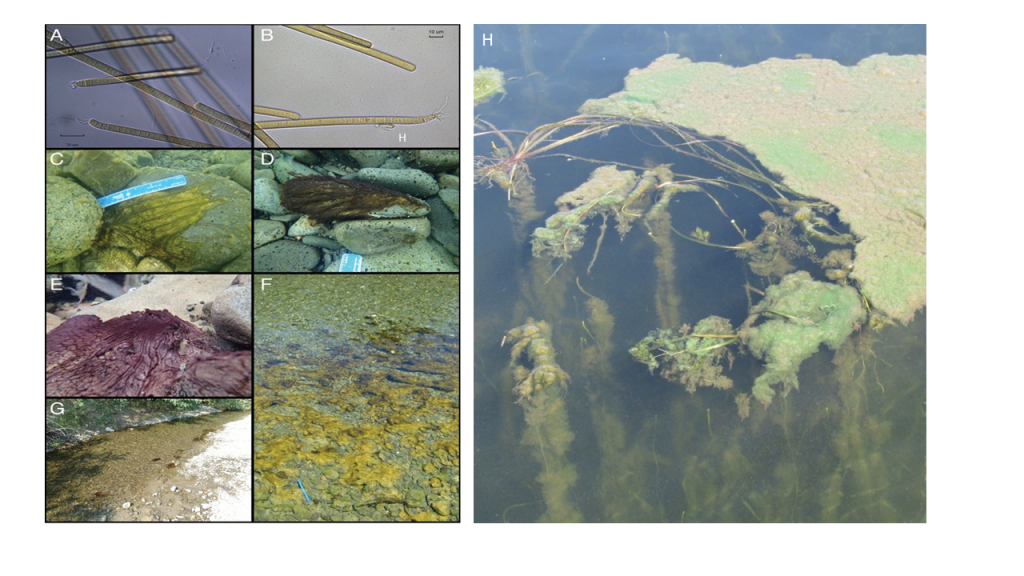
Figure 4‑10. Attached and benthic cyanobacteria. Phormidium in the Eel River: micrographs of Phormidium cells (400x, A and B), underwater photographs of Phormidium (C, D, and E), looking down on brown or orange patches of Phormidium mats in the river (blue thermometer is 15 cm long), H-Lyngbya growing on aquatic plants and spreading over the surface.
Figure Source: A – G Bouma-Gregson, Kudela, and Power (2018), H – Angela Shambaugh, VT DEC. Used with permission.
Attached filamentous cyanobacteria can also be found as epiphytes (growing on aquatic plants or other algae) in lakes and ponds or in slow-flowing streams. A review by Quiblier et al. (2013) outlines how little we know regarding “distribution, toxin production, and species composition” of benthic or attached cyanobacteria occurring in streams where cyanotoxins have been detected. Unless HCBs in these locations are visible, it is difficult to determine where and for what toxins to sample and test.
Examples of approaches for monitoring benthic mats include:
- Gaget et al. (2020) developed a toolbox for sampling and monitoring benthic cyanobacteria
- USGS’ National Water-Quality Assessment Program protocols
- Maine’s protocols for sampling algae in wadeable rivers, streams, and freshwater wetlands (Danielson 2014)
- New Zealand’s Stream Periphyton Monitoring Manual (Biggs and Kilroy 2000)
- Section 3.1 of HCB-2, Developing a Monitoring Plan for Benthic Cyanobacteria
4.4.2.1 Periphyton Scrapes
In wadeable streams with hard substrates, samples are often collected by setting up transects through the riffle/run areas and collecting scrapes of a known area by using a template held in place on the stone and cleaned with a toothbrush or knife. Bouma-Gregson, Kudela, and Power (2018) used 3.8-centimeter-diameter PVC pipe to demarcate an area on cobbles to collect cyanobacterial mats. Micro/macroalgal material is composited and brought back to the laboratory for identification, chlorophyll, and toxin testing (Danielson 2014).
4.4.2.2 Rake Samples
Deep water bodies can be sampled for benthic HCBs by using a double-headed rake attached to a sampling pole or by using a weighted rake head attached to a rope (Hauxwell et al. 2010). The pole rake can be pulled along the bottom in a consistent pattern, covering a similar-sized area each time to better evaluate differences in the material brought up from site to site. The weighed rake head can be tossed overboard and then dragged along the bottom; this method, however, is more difficult to deploy in a consistent fashion at each site (Figure 4-11).
In deep water, it can be difficult to see and target benthic HCBs. Rake samples can be kept separate or composited. Material can be used for cyanobacteria identification, cell counts, pigments, cyanotoxins, and genetic analyses. Rake results are not easily quantified since the area sampled differs with each rake toss. Smith et al. (2019) studied the spatial, temporal, and between-site variation of Microseira wollei in a New York lake using rake collections. (Poirier-Larabie et al. 2020) used this method to evaluate cyanotoxin release from benthic mats of Microseira wollei.

Figure 4‑11. Rake sampling can be used for benthic cyanobacteria.
Source: Kellie Merrell, Vermont Department of Environmental Conservation (A); Hauxwell et al. (2010) (B,C). Used with permission.
4.4.2.3 Ponar Samples
Ponar samplers are mechanical dredges used to sample bottom substrates like sand, gravel, or clay. They come in two sizes: petite and standard. Ponar samplers are usually deployed from boats and, so long as they can penetrate the substrate, collect HCBs from a standard-sized area (Cavanagh et al. (Undated)). Similar to rake tosses, it is difficult to see and target benthic HCBs in deep water. Material collected by Ponar dredge can be used for cyanobacteria identification, cell counts, pigments, cyanotoxins, and genetic analyses. Results are reported on an areal basis (for example, cells/m2). Rodusky et al. (2005) found that the results obtained by the rake method and the Ponar sampler compared favorably to each other.
4.4.2.4 Artificial Substrates
Artificial substrates such as microscope slides or ceramic or plastic plates can be placed in water bodies for a period of time that allows for periphyton communities, including cyanobacteria, to grow (see Gaget et al. 2020). These substrates are usually easier to process and produce data that can be quantified on an areal basis, such as cells/cm2. The accumulated biomass can be analyzed for cyanobacteria identification, cell counts, pigments, cyanotoxins, and genetic analyses.
4.4.2.5 Scuba and Snorkeling
In deeper waters, it can be difficult to target patchy growth of benthic cyanobacteria using rakes or Ponar samplers. Scuba or snorkeling allows you to get closer to the material and more easily collect a targeted sample. Since diving and snorkeling may increase the opportunity for contact with cyanobacteria or cyanotoxins, safety protocols should be developed for this approach. For more information, see Spoo-Chupka, Young, and Fadness (2019).
4.4.3 Sampling for Cyanotoxins
Any of the methods used to collect planktonic or benthic cyanobacteria will provide a sample that can be tested for the presence of cyanotoxins in the laboratory by the methods shared in Table 4-2. Most analyses will require a pre-analysis extraction process to separate cyanotoxins from the cells. Depending on the method of collection and preparation, results may be reported as µg/L or as µg/mg of dried mat.
Solid Phase Adsorption Toxin Tracking (SPATT) samplers use a variety of absorbent resins to capture and retain cyanotoxins (and many other kinds of chemicals) dissolved in the water (Figure 4-12). SPATT samplers can be deployed at single or multiple locations in a water body and collected at defined time intervals. As water flows through the sampler, extracellular cyanotoxins bind to the resin. After the samplers are removed from the water body, cyanotoxins are released from the resin by an extraction procedure and analyzed in the laboratory (Roué, Darius, and Chinain 2018).
It is difficult to calculate precise toxin concentrations using SPATT samplers, and they are primarily used for presence/absence and maximum production determinations. SPATT samplers are relatively inexpensive to make and deploy; however, cyanotoxin analysis, typically by mass spectrometry, is required and raises the overall cost of this sampling method. Examples of SPATT use include Wood, Holland, and MacKenzie (2011) and Howard et al. (2017).

Figure 4‑12. Deployment of 2.5-inch SPATT sampler in the North Fork Virgin River, Zion National Park, to evaluate cyanotoxins associated with a benthic mat.
Source: Robyn Henderek, National Park Service. Used with permission.
4.5 Water Quality Monitoring to Support Cyanobacteria Management
You may want to monitor additional water quality parameters known to be drivers of HCB development as part of your HCB management plan (Section 3.4). Common parameters include ambient water temperature, pH, macro- and micronutrient levels (usually forms of phosphorus and nitrogen), water transparency, and dissolved oxygen (Figure 4-13). Tracking some of these key parameters may provide early indication of increasing cyanobacteria concentrations. These data may also help evaluate the success of management activities intended to reduce the frequency and severity of cyanobacterial blooms. Your monitoring plan should specify required methods and detection limits that will support your monitoring and cyanobacteria management goals. Several guides are available for more information on the collection of these commonly measured indicators of water quality, including:
- USGS’ National Field Manual for the Collection of Water-Quality Data
- The National Park Service’s Lake Monitoring Field Manual (Nevers and Whitman 2002)
- USEPA’s water quality monitoring resources
- QAPPs for lake monitoring programs in your area and your state’s water quality monitoring guides and resources. Examples include:
- Vermont Water Quality Division’s Field Methods Manual (VTANR 2012)
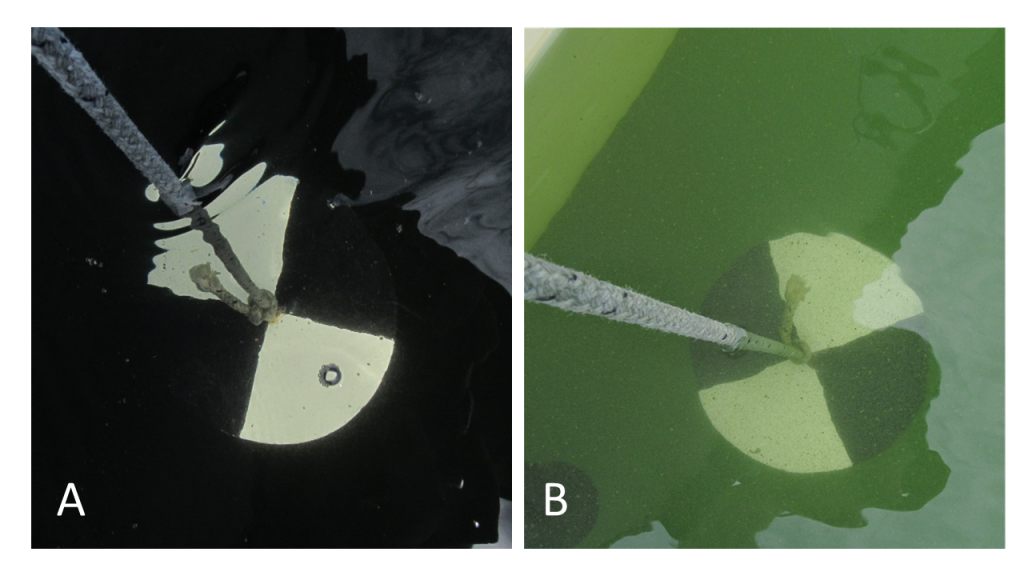
Figure 4‑13. Secchi discs are a common tool used to measure water transparency. Secchi measurements are often used to evaluate changes in phytoplankton, including cyanobacteria, over time. These images show clear water (A) and a cyanobacteria bloom event (B).
Source: Vermont Department of Environmental Conservation. Used with permission.
Long-term monitoring is especially important for water bodies where blooms are common events and could result in significant public exposure or economic losses. Cyanobacteria respond to several environmental factors (see Section 3.3.2), including nutrients, light levels, water temperature, and flow rates (Newcombe et al. 2010). Unusually severe spring or summer rain events, for example, are good indicators of increased nutrient loading from the watershed that could lead to HCBs in a few weeks. It is useful for drinking water facility operators to be knowledgeable about their source water susceptibility to HCBs, typical cycles experienced by their water body over the course of a summer, and changes in the watershed that may affect source water quality. A long-term record, even something as simple as photographs, can help beach managers interpret what they are seeing and what might lie ahead. You can gain valuable time to prepare by being able to recognize when conditions in your water body begin to deviate from what you expect. See our Section 5 to learn more.
4.5.1 Monitoring Platforms and Emerging Monitoring Techniques
Aquatic environments are very diverse, both across the landscape and within water bodies themselves. Research institutions have long employed high-frequency monitoring equipment and platform-based sensors to better capture the changes that occur across very short time frames (Figure 4-14). The costs and computer processing power needed to implement this type of monitoring often meant that these methods were available to research institutions and industry only. Now, however, platforms and sensors have become more affordable, and their careful placement can provide the information needed to inform HCB event response. Telecommunication systems can send data to a central processing location to enable near-real-time interpretation on public-facing websites and apps. An integrated monitoring approach including periodic lake-wide sampling and high-frequency monitoring at select locations may be a powerful addition to your cyanobacteria management toolbox.

Figure 4‑14. High-frequency monitoring buoy deployed at Lake Hopatcong, NJ.
Source: New Jersey Department of Environmental Protection. Used with permission.
Examples of how automated field sampling is being used for cyanobacteria monitoring include:
- USGS’ cyanobacteria monitoring in the Finger Lakes region of New York
- USEPA’s Charles River monitoring buoy
- USGS’ real-time monitoring data for Lake Hopatcong, New Jersey
- Research in Switzerland using in situ sensors and underwater cameras
4.6 Examples of Recreational and Drinking Water Monitoring Approaches for Cyanobacteria
Many states have some level of assessment to evaluate HCBs and protect public health. A number of states have developed routine recreational monitoring approaches to inform public health response and communicate cyanobacteria conditions to the general public. The USEPA and NALMS web pages maintain lists of cyanobacteria monitoring programs by state or region.