Appendix C. Management Strategy Fact Sheets
C.1 Introduction
This Section provides descriptions of management strategies presented in Table 6-1, evaluated for effectiveness, advantages, limitations, relative cost, and regulatory and policy considerations. Each fact sheet can stand alone and is intended to provide guidance and technical background to evaluate the use of a given management strategy in a harmful cyanobacterial bloom (HCB)–affected water body.
A summary of cost information for selection of management strategies is included in this guidance as Appendix C.2. The tables within each fact sheet detail relative cost ($, $$, $$$) per growing season to implement and maintain the strategy.
We necessarily limited this review to methods that are used in contemporary settings and have support from peer-reviewed literature. Some notable methods that were considered, but not reviewed in full, are included as Abridged Strategies in this appendix. Methods that are considered outdated or have only a very narrow range of applicability, as well as those that have only anecdotal support or endorsement from commercial providers, are not addressed in this document.
ACIDIFICATION
In-lake Prevention Strategy
Limited Supporting Field Data
The acidification of freshwater aquatic systems, either by surface discharge or by precipitation, has been noted as an issue of increasing environmental concern (Graham, Arancibia-Avila, and Graham 1996). In normal freshwater systems, an average pH is usually between 6.5 and 9.0 (USEPA 1986). Aquatic organisms, including cyanobacteria that may cause HCBs, exist in a variety of environments over wide pH ranges. However, these organisms have a tolerance that, once a pH shift occurs, may impact their ability to function and survive. Ecologists who have surveyed acidified lakes noted that cyanobacteria are often absent in benthic habitats where the pH is less than 4.0 and mildly acidic lakes with pH ranges of 5.0 to 6.0 (Brock 1973). Researchers proposed that shifting the pH into an acidic environment could control or eliminate cyanobacterial blooms (Klemer et al. 1996). In a recent review (Triest, Stiers, and Van Onsem 2016), data from mesocosm experiments indicate that the addition of CO2 to a pH around 7.0 kept cyanobacterial biomass low (Tessier et al. 2011 in Triest, Stiers, and Van Onsem 2016). Similarly, following biomanipulation of Lake Vesijärvi in Finland, Keto and Tallberg (2000) suggested that low pH may have prevented cyanobacterial dominance, and Peretyatko et al. (2012) list low pH as one parameter that limits cyanobacterial growth in hypereutrophic ponds.
Evidence of acidification occurring naturally has been reported. Planktonic species of cyanobacteria disappeared from the epilimnion (upper layer of water) in Little Rock Lake, Wisconsin, as the pH fell to 5.2 (Klemer et al. 1996). In controlled studies, blooms in experimental lakes remained dominated by cyanobacteria until the pH dropped below 5.2, at which point filamentous green algae became most abundant for a limited time (Turner et al. 1995). This pattern of acidification does not seem to be universal, however, as succession in the same lake showed a shift of Anabaena spp. and Lyngbya spp. to colonial species of Merismopedia and Chroococcus. This shift in species is consistent with other field observations that low pH seems to select for cyanobacteria that do not regulate their buoyancy by gas vesicles: As pH decreased from 5.9 to 5.1, the abundance of cyanobacteria that form gas vesicles decreased, while abundance of those without gas vesicles increased (Findlay and Kasian 1986).
There is limited applied data (see Teissier et al. 2011 mesocosm results above) to suggest that artificially acidifying water will prevent or control an ongoing HCB. Experimental acidification has been studied in a number of benchtop and laboratory assays that used bubbled CO2 to artificially lower the pH while the cells were growing under optimal conditions. These studies had results similar to the field data above—that growth of targeted species of cyanobacteria was adversely affected starting at pH <6.0 (Wang et al. 2011).
While the exact method of action is not known, acidification could physiologically inhibit cyanobacteria growth or adversely affect any number of biological processes the cyanobacteria use. Some laboratory observation data have highlighted that low pH inhibits important cellular functions, such as CO2 concentrating mechanisms. It has also been observed that low pH causes cyanobacterial cells to expend high levels of energy to maintain optimal intracellular pH range for metabolic processes and that low pH causes cells to build up carbonic acid, which can interfere with photosynthesis (Mangan et al. 2016).
EFFECTIVENESS
- Water body type: Pond, lake/reservoir
- Depth: Shallow
- Surface area: Small
- Any trophic state
- Any mixing regime
- Any water body use
NATURE OF HCB
- Cyanobacteria species that use gas vesicles to regulate buoyancy
- Unknown interaction with cyanotoxins
- Effects on all aquatic species, including off-target organisms
- Prevention strategy
ADVANTAGES
- Field observations note that potentially problematic cyanobacteria species are absent in acidified environments.
- Limited experimental data show that artificially lowering pH causes gas-vesicle-dominated cyanobacteria to die.
LIMITATIONS
- There are few full-scale studies (on entire ecosystem impact) on artificially lowering pH.
- Limited field data noted that while gas-vesicle-forming species disappeared, species that do not form gas vesicles were able to grow in their place.
- Laboratory studies that bubbled CO2 were conducted on pure cultures under non-field conditions.
COST ANALYSIS
Cost analysis per growing season: Acidification
| ITEM | RELATIVE COST PER GROWING SEASON |
| Material | $$ |
| Personal Protective Equipment | $ |
| Equipment | $$ |
| Machinery | $ |
| Tools | $ |
| Labor | $ |
| O&M Costs | $$ |
| Delivery | $ |
| OVERALL | $$ |
Adding CO2 to a water body will not necessarily shift the pH adequately to prevent or disrupt blooms of cyanobacteria, as pH depends on a variety of ambient conditions. Previous studies on acidification have pumped CO2 into vats of sample water using tanks of liquid CO2, which can be readily acquired from various vendors. The cost of this method will depend partly on whether the multiple bubble lines spanning a lake are derived from one tank or from multiple tanks. Multiple tanks can be spaced roughly 10 acres apart with individual bubblers.
REGULATORY AND POLICY CONSIDERATIONS
Implementation of acidification equipment may require installation of temporary tubing as well as investment in infrastructure to maintain and support the tools and supplies needed to maintain and monitor the supply of the bubbling system. Monitoring lake pH should be embedded in the treatment, as there is no known quantified relationship between the volume of gas added and the response of small lakes. Off-target effects are possible to fish and other aquatic life that may be impacted by the sudden shift in pH. Additional concerns about mobilization and immobilization of various metals should be considered and will depend on the chemistry of the water body and sediment. Various regulatory entities may prohibit shifts in pH more than 1 unit above or below typical background levels to minimize off-target effects of treatment. Applicable state water quality criteria must also be considered.
REFERENCES
Brock, Thomas D. 1973. “Lower pH limit for the existence of blue-green algae: evolutionary and ecological implications.” Science 179 (4072):480-483. doi: 10.1126/science.179.4072.480.
Findlay, D. L., and S. E. M. Kasian. 1986. “Phytoplankton community responses to acidification of lake 223, experimental lakes area, northwestern Ontario.” Water, Air, and Soil Pollution 30 (3):719-726. doi: 10.1007/BF00303337.
Graham, James M., Patricia Arancibia-Avila, and Linda E. Graham. 1996. “Effects of pH and selected metals on growth of the filamentous green alga Mougeotia under acidic conditions.” Limnology and Oceanography 41 (2):263-270. doi: 10.4319/lo.1996.41.2.0263.
Keto, Juha, and Petra Tallberg. 2000. “The recovery of Vesijärvi, a lake in southern Finland: water quality and phytoplankton interpretations.” Boreal Environmental Research 5.
Klemer, Andrew R., John J. Cullen, Michael T. Mageau, Kathryn M. Hanson, and Richard A. Sundell. 1996. “Cyanobacterial buoyancy regulation: the paradoxical roles of carbon.” Journal of Phycology 32 (1):47-53. doi: 10.1111/j.0022-3646.1996.00047.x.
Mangan, N. M., A. Flamholz, R. D. Hood, R. Milo, and D. F. Savage. 2016. “pH determines the energetic efficiency of the cyanobacterial CO2 concentrating mechanism.” Proc Natl Acad Sci U S A 113 (36):E5354-62. doi: 10.1073/pnas.1525145113.
Peretyatko, Anatoly, Samuel Teissier, Sylvia De Backer, and Ludwig Triest. 2012. “Classification trees as a tool for predicting cyanobacterial blooms.” Hydrobiologia 689 (1):131-146. doi: 10.1007/s10750-011-0803-4.
Triest, Ludwig, Iris Stiers, and Stijn Van Onsem. 2016. “Biomanipulation as a nature-based solution to reduce cyanobacterial blooms.” Aquatic Ecology 50 (3):461-483. doi: 10.1007/s10452-015-9548-x.
Turner, M. A., D. W. Schindler, D. L. Findlay, M. B. Jackson, and G. G. Robinson. 1995. “Disruption of littoral algal associations by Experimental Lake acidification.” Canadian Journal of Fisheries and Aquatic Sciences 52 (10):2238-2250. doi: 10.1139/f95-815b.
USEPA. 1986. “Quality Criteria for Water, 1986 EPA 440/5-86-001.” Washington, D. C.: U. S. Environmental Protection Agency, Office of Water. https://www.epa.gov/sites/production/files/2018-10/documents/quality-criteria-water-1986.pdf.
Wang, X., C. Hao, F. Zhang, C. Feng, and Y. Yang. 2011. “Inhibition of the growth of two blue-green algae species (Microsystis aruginosa and Anabaena spiroides) by acidification treatments using carbon dioxide.” Bioresour Technol 102 (10):5742-8. doi: 10.1016/j.biortech.2011.03.015.
ARTIFICIAL CIRCULATION AND MECHANICAL MIXERS
In-lake Prevention Strategy
Substantial Supporting Data
Artificial circulation and mechanical mixers have been successfully used in lakes and reservoirs as physical controls to increase oxygen concentrations in bottom waters of lakes and reservoirs and to destratify the water column to remove the optimal habitat for buoyant cyanobacteria (Beutel and Horne 1999, Bormans, Marsálek, and Jancula 2015, Visser et al. 2016). Artificial circulation and mechanical mixers completely mix a stratified lake or reservoir, redistributing oxygen and nutrients throughout the water column (Hudson and Kirschner 1997).
Generally, these techniques also cause a temperature increase in the deep layers and a temperature decrease in the upper layers, while increasing spatial phytoplankton distribution and concentration due to an increase in the limiting nutrient entrained from the hypolimnion or resuspended from the sediments (Visser et al. 2016).
The two most common types of destratification are air injection and mechanical mixing (Hudson and Kirschner 1997). Air injection is a “bottom-up” approach that quickly pumps air to the bottom of the lake so that it will rise and carry the water from the hypolimnetic layers to the top layer (Hudson and Kirschner 1997). Mechanical mixing uses a “top-down” approach wherein a rotating propeller in the surface layers pushes the water downward, displacing bottom waters to the surface, where they are reoxygenated by the atmosphere (Hudson and Kirschner 1997). Popular commercially available models are powered by solar panels. Although artificial circulation is beneficial for oxygen and nutrient redistribution, the ecological effects on plant and animal life of destratifying a lake are not always predictable and could potentially be harmful (Hudson and Kirschner 1997).
EFFECTIVENESS
- Water body type: Lake/reservoir
- Any surface area
- Depth: Deep; requires large hypolimnion; avoid in shallow, unstratified systems
- Any trophic state, but typically most effective in eutrophic systems
- Mixing regime: Meromictic, monomictic, or dimictic
- Any water body use
- Watershed loading levels will impact effectiveness
NATURE OF HCB
- Repeating HCBs
- Toxic and nontoxic HCBs; effective for cyanotoxins
- Targets all algal species
- Prevention strategy
These physical controls are most effective in systems that have or are expected to experience extensive, sustained nutrient and sediment loading and require remediation beyond periodic intervention strategies to protect the water quality and ecosystem (Bormans, Marsálek, and Jancula 2015). Often artificial circulation and mechanical mixers are used in conjunction with watershed controls and algaecide treatments (Bormans, Marsálek, and Jancula 2015, Moore and Christensen 2009, Visser et al. 2016). Artificial circulation and mechanical mixing methods can cause a change in composition from cyanobacterial dominance to green algae and diatoms if the water body is deep enough to limit light availability (Bormans, Marsálek, and Jancula 2015, Visser et al. 2016).
ADVANTAGES
- No waste or by-products produced
- Readily available equipment
- Successful full-scale implementation
- Reported water quality and ecological benefits
- Indiscriminate of algae species
- In areas around the devices, habitats supporting cyanobacteria are lost
LIMITATIONS
- High installation costs
- High operational costs associated with use
- Needs infrastructure (electricity, boat ramp, etc.)
- Limited scalability
- Potential unintended water quality impacts
- Potential unintended biological impacts
- Potential aesthetic concerns
Successful deployment of artificial circulation and mechanical mixers can establish a diatom population, allow this diatom population to persist longer, and remove limiting nutrients from the water column so that fewer nutrients are available in the epilimnion for cyanobacterial growth (Bormans, Marsálek, and Jancula 2015). Unanticipated biological effects associated with destratification may result from mechanical mixing due to sudden water quality and chemistry changes; however, some biological and ecological benefits may also result from this process (Pastorok, Ginn, and Lorenzen 1981). Artificial circulation may allow for deeper zooplankton distribution and refuge from predators in the dark bottom waters during the day (McComas 2003). In addition, the expanded aerobic environment may enhance growth and expansion of cold-water fish habitat and population due to increased oxygen concentrations, increased visibility, and greater zooplankton density (Rieberger and BC Environment 1992).
COST ANALYSIS
Cost analysis per growing season: Artificial circulation and mechanical mixers
| ITEM | RELATIVE COST PER GROWING SEASON |
| Material | $$ |
| Personal Protective Equipment | $ |
| Equipment | $$$ |
| Machinery | $$$ |
| Labor | $$ |
| O&M Costs | $$$ |
| OVERALL | $$$ |
The costs of installing and maintaining an artificial circulation or mechanical mixing system are relatively high (mostly due to operating costs for successful applications) and dependent on the type of equipment and local power rates (Bormans, Marsálek, and Jancula 2015). The use of photovoltaic technologies and availability of brand-named, solar-powered mechanical mixers may help mediate power costs. Some cost estimates can be found in Appendix C.2.
REGULATORY AND POLICY CONSIDERATIONS
Before implementing a management action, you should establish a cause–effect linkage between the problem and the proposed management approach (Hickey and Gibbs 2009, USEPA 2000). Because multiple stressors and environmental factors combine to cause the effects observed in aquatic ecosystems, an integrated approach with multiple management measures is often required to holistically address ecological issues in lakes. The decision to introduce an artificial circulation or mechanical mixing system should be based on a thorough understanding of the factors contributing to recurrent blooms and preliminary research to establish that a destratification approach is a feasible option for reducing the frequency and severity of HCBs.
This assessment must also include social and cultural values that need to be considered on a case-by-case basis with public and multi-agency consultation, which could uncover concerns with a specific product or approach. The selection and decision-making process may need to be modified accordingly. Any supplementary watershed controls or algaecide treatments must comply with policies and regulations as enacted by the appropriate oversight agency or authority. For some lakes, additional approval may be required from the U.S. Fish and Wildlife Service and the National Oceanic and Atmospheric Administration’s National Marine Fisheries Service under the Endangered Species Act (ESA) if endangered, threatened, or otherwise special status species are present, or if the lakes are in conservation land (USFWS 2020). Special consideration for protection of native or indigenous species may be made.
REFERENCES
Beutel, Marc W., and Alex J. Horne. 1999. “A review of the effects of hypolimnetic oxygenation on lake and reservoir water quality.” Lake and Reservoir Management 15 (4):285-297. doi: 10.1080/07438149909354124.
Bormans, Myriam, Blahoslav Marsálek, and Daniel Jancula. 2015. “Controlling internal phosphorus loading in lakes by physical methods to reduce cyanobacterial blooms: a review.” Aquatic Ecology 50. doi: 10.1007/s10452-015-9564-x.
Hickey, Christopher W., and Max M. Gibbs. 2009. “Lake sediment phosphorus release management—Decision support and risk assessment framework.” New Zealand Journal of Marine and Freshwater Research 43 (3):819-856. doi: 10.1080/00288330909510043.
Hudson, Holly, and Bob Kirschner. 1997. “Lake aeration and circulation.” Lake Notes. doi: https://www2.illinois.gov/epa/Documents/epa.state.il.us/water/conservation/lake-notes/lake-aeration.pdf.
McComas, Steve. 2003. Lake and pond management guidebook. Boca Raton, FL: Lewis Publishers.
Moore, Barry C., and David Christensen. 2009. “Newman Lake restoration: A case study. Part I. Chemical and biological responses to phosphorus control.” Lake and Reservoir Management 25 (4):337-350. doi: 10.1080/07438140903172907.
Pastorok, R., T. Ginn, and M. Lorenzen. 1981. “Evaluation of Aeration/Circulation as a Lake Restoration Technique. EPA/600/3-81/014.” Washington, D. C. : U. S. Environmental Protection Agency. https://cfpub.epa.gov/si/si_public_record_Report.cfm?Lab=ORD&dirEntryID=45821.
Rieberger, K., and BC Environment. 1992. “Metal Concentrations in Fish Tissue from Uncontaminated B.C. Lakes.” Victoria: Water Quality Section. https://books.google.com/books?id=OLG3AAAACAAJ.
USEPA. 2000. “Stressor Identification Guidance Document EPA 822-B-00-025.” U. S. Environmental Protection Agency, Office of Water. https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=20685.
USFWS. 2020. “Endangered Species Laws and Policies.” U. S. Fish and Wildlife Service, Ecological Services. https://www.fws.gov/sites/default/files/documents/endangered-species-act-accessible.pdf.
Visser, Petra M, Bas W Ibelings, Myriam Bormans, and Jef Huisman. 2016. “Artificial mixing to control cyanobacterial blooms: a review.” Aquatic Ecology 50 (3):423-441.
BARLEY AND RICE STRAW
In-lake Prevention Strategy
Substantial Supporting Field Data
Barley straw (Hordeum vulgare) has been used for over four decades to prevent the growth of cyanobacteria. Initial reports showed widespread success in the United Kingdom, and barley straw deployment has spread to the United States in the past 20 years (Sellner and Rensel 2018). Decomposition of barley straw leads to the breakdown of lignin-containing cell walls within the straw. Lignin decomposition produces two types of residues that limit cyanobacterial growth. Some are specific compounds that individually inhibit cyanobacteria, while others yield strong oxidizing agents that rapidly reduce cell viability. For details and examples, please see Huang et al. (2015), Matthijs et al. (2012), Pillinger, Cooper, and Ridge (1994), Ridge and Pillinger (1996), Xiao et al. (2010), and Xiao et al. (2014).
The general procedure is as follows: 1–1.5 months prior to an expected HCB, stake or otherwise secure <1-year-old, fungicide-free bales of barley straw into the littoral zone of ponds, lakes, or incoming streams. Bales should be applied at a rate of 7 bales/acre, with several bales saved to deploy halfway through the summer. Bales should be reapplied each year thereafter, again saving some bales for mid-summer deployment. Ranges for barley straw treatment of cyanobacteria in other systems are 6–50 mg barley straw/L in longer residence time waters, such as lakes or reservoirs (Sellner and Rensel 2018).
EFFECTIVENESS
- Water body type: Pond, lake/reservoir, bay/estuary
- Any surface area or depth
- Any trophic state
- Any mixing regime
- Water body uses: Recreation, drinking water
NATURE OF HCB
- All HCB types in ponds to estuaries
- Singular or repeating HCBs
- Toxic and nontoxic HCBs
- Prevention strategy
This technique (7 bales/acre) is effective for most ponds, lakes, reservoirs, and low-salinity estuarine areas and is even more effective if enriched with fungi to aid in lignin decomposition (Sellner et al. 2015). There are some concerns about tannin removal in drinking water facilities from decomposing straw. This technique will not work if applied after the HCB has appeared, and it will not be as effective if the bales are placed in low-light or dark areas. Straw is used in eutrophic systems where blooms have historically occurred; hence, their decomposition results in minimal nutrient additions relative to available levels for bloom growth.
ADVANTAGES
- Effective for most HCBs
- Prevents HCBs and, therefore, any toxin accumulations
- Used in many areas
- Cost is low if bales are purchased from a farmer
- Securing bales along the shoreline is easy
- No impact on submersed plants or fish
- Is an unregistered algaecide, so may be deployed by individuals, groups, etc., but not by licensed applicators
LIMITATIONS
- Will work on most systems, but very large lakes would require significant staff effort for bale deployment
- Possible open-water obstruction, so the U.S. Army Corps of Engineers may need to be contacted
- Straw decomposition products include tannins, a concern for removal in drinking water facilities
- A small mid-summer bale addition may be required
- Some biological oxygen demand accompanies straw decomposition, possibly affecting dissolved oxygen levels, but this is overcome by nearshore deployment, where reoxygenation is continuous
- Some lake organizations object to bale use due to aesthetics

Figure C-1. (A) Barley straw lining a stream entering an HCB-dominated lake in eastern Maryland and (B) along the shoreline of a brackish lagoon in Chesapeake Bay.
Source: A–Place, B–K. Sellner. Used with permission.
Other similar options are found in Effiong et al. (2020): Rice straw inhibits Microcystis aeruginosa in the laboratory (Park et al. 2006), was used effectively in Nile tilapia ponds (Eladel, Abd-Elhay, and Anees 2019, Shahabuddin et al. 2012), and inhibited Anabaena in laboratory experiments (Eladel et al. 2019). Using lake water in aquaria, Tomasko, Britt, and Carnevale (2016) reported that dried cypress leaves at 1.51 g/39 L were more inhibitory to cyanobacteria than equal additions of barley straw.
CASE STUDY EXAMPLES
Williston Lake, Denton, Maryland, United States: 500 barley straw bales were deployed over 67 acres of an incoming stream and shoreline of Williston Lake from April to May while the lake was partially drained, resulting in the lake remaining free of Microcystis aeruginosa. Microcystin and anatoxin-a concentrations were below recreational exposure levels in the first year, followed by absence of the species and toxins in subsequent years (Sellner et al. 2015).
Ponds, drainage ditches, and lakes, United Kingdom and Ireland: Barley straw was effective in reducing Oscillatoria agardhii from 10,000 filaments/mL to nondetectable levels in a 6-ha lake after 3 weeks of exposure. Lake managers for 29 other water bodies indicated dramatic cyanobacteria reductions following barley straw additions (Newman and Barrett 1993).
Potable water reservoir, Aberdeen, Scotland: Approximately twice/year barley straw treatment (6–28 g/m3) of a reservoir from 1993 to 1998 substantially reduced cyanobacteria (Barrett, Littlejohn, and Curnow 1999).
Derbyshire Reservoir, United Kingdom: Cyanobacteria were significantly reduced when 50 g/m3 and 25 g/m3 of barley straw were added to a disused UK water supply reservoir (Everall and Lees 1996, 1997).
Pond, Dublin, Ireland: Barley straw additions (25–50 g/m2) to the pond at the Tolka Valley Park in Finglas, Dublin, prevented growth of Lyngbya mats (Stack and Zhao 2014).
COST ANALYSIS
Costs for fungicide-free barley straw bales from farmers are inexpensive relative to retail prices from landscape or pond supply companies, where they can be five to 10 times more expensive. Implementation requires labor to secure bales in the littoral zone and may require a small mid-summer bale addition.
Cost analysis per growing season: Barley straw
| ITEM | RELATIVE COST PER GROWING SEASON |
| Material | $ |
| Equipment | $ |
| Labor | $ |
| OVERALL | $ |
REGULATORY AND POLICY CONSIDERATIONS
The only limitations for bale deployment are aesthetics (viewsheds) and boating obstructions if bales are secured in open water.
REFERENCES
Barrett, P. R. F., J. W. Littlejohn, and J. Curnow. 1999. “Long-term algal control in a reservoir using barley straw.” Hydrobiologia 415 (0):309-313. doi: 10.1023/A:1003829318450.
Effiong, Kokoette, Jing Hu, Caicai Xu, Tao Tang, Haomin Huang, Jiangning Zeng, and Xi Xiao. 2020. “Sustainable utilization of agricultural straw for harmful algal blooms control: a review.” Journal of Renewable Materials 8:461-483. doi: 10.32604/jrm.2020.09111.
Eladel, Hamed, Mohamed Battah, Aida Dawa, Reham Abd-Elhay, and Doaa Anees. 2019. “Effect of rice straw extracts on growth of two phytoplankton isolated from a fish pond.” Journal of Applied Phycology 31 (6):3557-3563. doi: 10.1007/s10811-019-01766-0.
Eladel, Hamed Mohamed, Reham Abd-Elhay, and Doaa Anees. 2019. “Effect of rice straw application on water quality and microalgal flora in fish ponds.” Egyptian Journal of Botany 59 (1):171-184. doi: 10.21608/ejbo.2018.4852.1199.
Everall, N. C., and D. R. Lees. 1996. “The use of barley-straw to control general and blue-green algal growth in a Derbyshire reservoir.” Water Research 30 (2):269-276. doi: 10.1016/0043-1354(95)00192-1.
Everall, N. C., and D. R. Lees. 1997. “The identification and significance of chemicals released from decomposing barley straw during reservoir algal control.” Water Research 31 (3):614-620. doi: 10.1016/S0043-1354(96)00291-6.
Huang, Haomin, Xi Xiao, Anas Ghadouani, Jiaping Wu, Zeyu Nie, Cheng Peng, Xinhua Xu, and Jiyan Shi. 2015. “Effects of natural flavonoids on photosynthetic activity and cell integrity in Microcystis aeruginosa.” Toxins 7 (1):66-80. doi: 10.3390/toxins7010066.
Matthijs, H. C., P. M. Visser, B. Reeze, J. Meeuse, P. C. Slot, G. Wijn, R. Talens, and J. Huisman. 2012. “Selective suppression of harmful cyanobacteria in an entire lake with hydrogen peroxide.” Water Res 46 (5):1460-72. doi: 10.1016/j.watres.2011.11.016.
Newman, Jonathan, and P. R. F. Barrett. 1993. “Control of Microcystis aeruginosa by decomposing barley straw.” Journal of Aquatic Plant Management 31:203-206.
Park, M. H., M. S. Han, C. Y. Ahn, H. S. Kim, B. D. Yoon, and H. M. Oh. 2006. “Growth inhibition of bloom-forming cyanobacterium Microcystis aeruginosa by rice straw extract.” Lett Appl Microbiol 43 (3):307-12. doi: 10.1111/j.1472-765X.2006.01951.x.
Pillinger, J. M., J. A. Cooper, and I. Ridge. 1994. “Role of phenolic compounds in the antialgal activity of barley straw.” Journal of Chemical Ecology 20 (7):1557-1569. doi: 10.1007/BF02059880.
Ridge, Irene, and J. M. Pillinger. 1996. “Towards understanding the nature of algal inhibitors from barley straw.” Hydrobiologia 340 (1):301-305. doi: 10.1007/BF00012772.
Sellner, Kevin, Allen Place, Ernest Williams, Yonghui Gao, Elizabeth VanDolah, Michael Paolisso, Holly Bowers, and Shannon Roche. 2015. “Hydraulics and Barley Straw (Hordeum vulgare) as effective Treatment Options for a Cyanotoxin-Impacted Lake.” International Conference on Harmful Algae.
Sellner, Kevin, and J. E. Rensel. 2018. “Prevention, control, and mitigation of harmful algal bloom impacts on fish, shellfish, and human consumers.” In Harmful Algal Blooms: A Compendium Desk Reference, edited by Sandra E. Shumway, JoAnn M. Burkholder and Steven L. Morton, 435-492. Wiley-Blackwell.
Shahabuddin, A. M., M. Oo, Y. Yi, Dhirendra Thakur, Amrit Bart, and J. Diana. 2012. “Study about the effect of rice straw mat on water quality parameters, plankton production and mitigation of clay turbidity in earthen fish ponds.” World Journal of Fish and Marine Sciences 4:577-585. doi: 10.5829/idosi.wjfms.2012.04.06.6515.
Stack, John, and Yaqian Zhao. 2014. “Performance assessment of an integrated constructed wetland-pond system in Dublin, Ireland ” Journal of Water Sustainability 4 (1):13-26.
Tomasko, David, Mike Britt, and M. Carnevale. 2016. “The ability of barley straw, cypress leaves and L-lysine to inhibit cyanobacteria in Lake Hancock, a hypereutrophic lake in Florida.” Florida Scientist 79:147-157.
Xiao, X., H. Huang, Z. Ge, T. B. Rounge, J. Shi, X. Xu, R. Li, and Y. Chen. 2014. “A pair of chiral flavonolignans as novel anti-cyanobacterial allelochemicals derived from barley straw (Hordeum vulgare): characterization and comparison of their anti-cyanobacterial activities.” Environ Microbiol 16 (5):1238-51. doi: 10.1111/1462-2920.12226.
Xiao, Xi, Ying-xu Chen, Xin-qiang Liang, Li-ping Lou, and Xian-jin Tang. 2010. “Effects of Tibetan hulless barley on bloom-forming cyanobacterium (Microcystis aeruginosa) measured by different physiological and morphologic parameters.” Chemosphere 81 (9):1118-1123. doi: 10.1016/j.chemosphere.2010.09.001.
CLAY AND SURFACTANT FLOCCULATION
In-lake Intervention Strategy
Substantial Supporting Field Data
Flocculation is the use of added compounds to bind, inactivate, or sink harmful algae or cyanobacteria. After the strategy was implemented successfully in marine systems (Sengco and Anderson 2004), investigation began for use of this intervention to control freshwater cyanobacteria blooms (Pan et al. 2006, Zou et al. 2006). Research teams tested an acidified mixture of local sediments combined with surfactants like chitosan (crustacean shell derivative) and polyaluminum chloride (PAC), the latter commonly used as a coagulant in drinking water facilities) for cyanotoxin removal in Ohio. These proved effective in the flocculation and settling of HCB blooms and some of their associated toxins in a variety of water bodies, from ponds and lakes to brackish estuaries. A mixture of suspended sediment/PAC/chitosan to reach 100 mg soil/10 mg PAC/5 mg chitosan in a lake (Pan et al. 2011) followed by capping (covering) with local sands can remove the HCB and support growth of submersed grasses, which are effective nutrient and sediment traps and provide habitat for many juvenile fish (Pan, Chen, and Anderson 2011, Pan et al. 2019).
EFFECTIVENESS
- Any water body type
- Any surface area or depth
- Any trophic state
- Water body uses: Recreation, drinking water source
- If no capping is done, best if used in a system with high near-bottom flushing rates
NATURE OF HCB
- All HCB types
- Singular or repeating HCBs
- Toxic and nontoxic HCBs; can remove cyanotoxins as well as cells
- Intervention strategy
This technique is effective for most ponds, lakes, reservoirs, and saline environments. The surfactant chitosan can be dissolved thoroughly in 0.1 N HCl or dilute vinegar (acetic acid). Because the flocculated material settles, capping can prevent resuspension and bloom return. If the capping material is mixed with seeds of submersed grasses, HCB areas can revegetate (Pan, Chen, and Anderson 2011). If capping is not employed in deep, stratified systems, decomposition of settled material can promote oxygen reduction and associated problems with hypoxia, anoxia, and loss of habitat and induce high nutrient fluxes from the sediments.
ADVANTAGES
- Effective for most HCBs
- Removes cells and toxins
- Used in many areas
- Easy spray dispersal
LIMITATIONS
- May require permit for dispersal
- Requires large volumes of acidic surfactants and sediments and high-volume pumps
- Scalable, but costly with increasing HCB area
- May impact bottom oxygen levels and benthic fauna and increase nutrient fluxes. Repeated additions may be required

Figure C-2. Spraying of local soils and chitosan in China.
Source: G. Pan, Nottingham Trent University, UK. Used with permission.
CASE STUDY EXAMPLES
Xuanwu Lake, China: Peak abundances of Microcystis aeruginosa exceeded 2.7×107 cells/mL in the summer of 2005. Through intermittent spraying of modified clays (3–5 tons/km2/d or 30–50 tons/km2 over 10 days), M. aeruginosa was reduced to 6×103 cells/mL and dissolved microcystin was reduced to <0.01 μg/L from 0.03–0.62 μg/L. Removal of flagellated algal blooms required rigorous sediment preparations and costly infrastructure for dispersal (Yu et al. 2017).
South Korea: Clays and electrolysis of local seawater have been used to remove toxic dinoflagellates in aquaculture areas (Park et al. 2013).
COST ANALYSIS
Relative cost per growing season: Clay and surfactant flocculation
| ITEM | RELATIVE COST PER GROWING SEASON |
| Material | $$ |
| Personal Protective Equipment | $ |
| Equipment | $$–$$$ |
| Machinery | $$ |
| Tools | $ |
| Labor | $ |
| O&M Costs | $$ |
| OVERALL | $$–$$$ |
In one study (Pan et al. 2019), costs ranged from $148/acre to $245/acre with two different surfactants and sediments; with capping, the cost increases to $3,648/acre to $8,197/acre. Costs for sediment, surfactants, pumps, and hosing can be high and are proportional to the treatment area. A boat may be required if the HCB cannot be treated from the shore.
CASE STUDY EXAMPLES
Lake Tai and Cetian Reservoir, China: Chitosan flakes were dissolved in 0.5% acetic acid (vinegar) and stirred until all the chitosan was dissolved; the solution was diluted with pond water to obtain a final concentration of 1 g/L before use. Based on lake acreage, the required volume of chitosan solution was mixed with the soil suspension (diluted using pond water) to make up a final concentration of 100 mg soil/L and 3 mg chitosan/L in the pond after spraying. For the Cetian Reservoir pond experiment, chitosan-PAC-modified local sediment (MLS) was prepared by adding dissolved PAC to chitosan-modified local soils to achieve a final concentration of 100 mg soil/L, 10 mg PAC/L, and 5 mg chitosan/L in the pond. In the latter, nutrient concentrations and chemical oxygen demand (COD) dramatically declined (Pan et al. 2019).
Tanxi Bay, Lake Tai, China: In 2012, approximately 16 kg of chitosan-MLS was sprayed into a 400 m2, 1.5-m-deep pond with a Secchi depth <5 cm. After treatment, the blooms were removed from the pond within 2 hours. Secchi depth (water clarity) increased to 1.5 m on the second day. The chlorophyll a concentration in the treated pond decreased from 85 µg/L to 13 µg/L and remained below this level for 20 days after the treatment. chlorophyll a in the control pond continually increased, reaching a concentration of 350 µg/L on day 20. Turbidity was reduced from 95 NTU to 5.3 NTU in the treatment pond, while it was maintained above 100 NTU in the control pond during the same period. COD and nutrient concentrations declined as well (Pan et al. 2019).
REGULATORY AND POLICY CONSIDERATIONS
Dispersing sediment may require a permit. If flocculation is not followed by capping, bottom impacts should be considered, including the smothering of bottom plants and animals, development of hypoxia/anoxia and associated loss of habitat for fish, and enhanced nutrient fluxes from bottom sediments that could exacerbate additional blooms.
REFERENCES
Pan, G, X. Miao, L. Bi, H. Zhang, L. Wang, L. Wang, Z. Wang, J. Chen, J. Ali, M. Pan, J. Zhang, B. Yue, and T. Lyu. 2019. “Modified local soil (MLS) technology for harmful algal bloom control, sediment remediation, and ecological restoration.” Water 11. doi: 10.3390/w11061123.
Pan, G., J. Chen, and D. M. Anderson. 2011. “Modified local sands for the mitigation of harmful algal blooms.” Harmful Algae 10 (4):381-387. doi: 10.1016/j.hal.2011.01.003.
Pan, G., H. Zou, H. Chen, and X. Yuan. 2006. “Removal of harmful cyanobacterial blooms in Taihu Lake using local soils. III. Factors affecting the removal efficiency and an in situ field experiment using chitosan-modified local soils.” Environ Pollut 141 (2):206-12. doi: 10.1016/j.envpol.2005.08.047.
Pan, Gang, Bo Yang, Dan Wang, Hao Chen, Bing-hui Tian, Mu-lan Zhang, Xian-zheng Yuan, and Juan Chen. 2011. “In-lake algal bloom removal and submerged vegetation restoration using modified local soils.” Ecological Engineering 37:302-308. doi: 10.1016/j.ecoleng.2010.11.019.
Park, Tae Gyu, Weol Ae Lim, Young Tae Park, Chang Kyu Lee, and Hae Jin Jeong. 2013. “Economic impact, management and mitigation of red tides in Korea.” Harmful Algae 30:S131-S143. doi: 10.1016/j.hal.2013.10.012.
Sengco, Mario, and Donald Anderson. 2004. “Controlling harmful algal blooms through clay flocculation.” The Journal of eukaryotic microbiology 51:169-72. doi: 10.1111/j.1550-7408.2004.tb00541.x.
Yu, Zhiming, Xiuxian Song, Xihua Cao, and Yang Liu. 2017. “Mitigation of harmful algal blooms using modified clays: Theory, mechanisms, and applications.” Harmful Algae 69:48-64. doi: doi.org/10.1016/j.hal.2017.09.004.
Zou, Hua, Gang Pan, Hao Chen, and Xianzheng Yuan. 2006. “Removal of cyanobacterial blooms in taihu lake using local soils. ii. effective removal of microcystis aeruginosa using local soils and sediments modified by chitosan.” Environmental pollution 141:201-5. doi: 10.1016/j.envpol.2005.08.042.
COPPER ALGAECIDES
In-lake Intervention and Prevention Strategy
Substantial Supporting Field Data
Copper algaecides have been used to treat problematic algae and cyanobacteria for more than a century due to their effectiveness (Moore and Kellerman 1905). As such, copper algaecides have been extensively evaluated, and numerous peer-reviewed publications have increased our understanding of copper algaecide efficacy, copper fate, and potential off-target aquatic life impacts (Calomeni, Rodgers, and Kinley-Baird 2014, Fitzgerald and Faust 1963, Gibson 1972, Iwinski et al. 2017, Kinley et al. 2017, Murray-Gulde et al. 2002). Cyanobacterial responses to copper algaecides are concentration dependent. At effective concentrations of copper algaecides, respiration and photosynthesis rates can be decreased, leading to a decrease in cell density (Calomeni, Rodgers, and Kinley-Baird 2014). At higher concentrations, copper algaecides impact cell integrity, causing cell lysis and decreased viability (Gibson 1972, Iwinski et al. 2016).
There are a variety of forms of copper algaecides, and cyanobacterial responses to these algaecides range as a function of innate cyanobacterial sensitivities (Calomeni, Rodgers, and Kinley-Baird 2014, Iwinski et al. 2017), abundances (Calomeni et al. 2018, Kinley et al. 2017), exposure durations (Calomeni et al. 2018), site characteristics (water hardness, alkalinity, conductivity, pH), and the copper-based algaecide applied (Fitzgerald and Faust 1963, Murray-Gulde et al. 2002). Copper algaecides include copper sulfate, acidified copper products, and chelated copper algaecides (copper ethanolamine, copper citrate, and copper gluconate). Copper algaecides have different trade names and are registered with the U.S. Environmental Protection Agency (USEPA) for treatment of excessive algae and cyanobacteria. The product’s label specifies how the compounds may be applied in lakes, reservoirs, ponds, irrigation canals, and other water bodies. To be effective, the algaecide must be applied so that the active ingredient contacts the problematic alga or cyanobacterium. Following an effective algaecide application, cell and population responses can be measured in as little as one day after treatment (Bishop and Rodgers 2011, Isaacs et al. 2013).
Copper algaecides are often applied when harmful algae and cyanobacteria achieve high densities, produce toxins, or produce taste and odor compounds that pose risks or interfere with the uses of water resources. The timing of algaecide treatments is often important to ensure treatment success and may limit potential adverse impacts of the cyanobacteria. A detailed management plan that includes monitoring of the cyanobacteria issue and explicit triggers for treatments with respect to a measured cyanobacterial cell density, cyanotoxin concentration, or taste and odor compound concentration (Calomeni et al. 2017) is useful for ensuring well-timed treatments for sites that experience recurring HCB issues.
EFFECTIVENESS
- Any water body type
- Any depth
- Surface area: Algaecide labels may specify applicable area (for example, maximum of half of the surface area of the water body can be treated at one time)
- Any trophic state
- Any mixing regime
- Water body uses: Algaecide labels will specify applicable uses
NATURE OF HCB
- Single or repeating HCBs
- Algal or cyanobacterial sensitivities to copper algaecides vary, but cyanobacteria are often more sensitive to copper algaecides than green algae
- Intervention and prevention strategy
ADVANTAGES
- More than a century of use and effectiveness in the United States
- Scalable
- Can be used to target specific problematic algal or cyanobacterial species
LIMITATIONS
- Because copper is a USEPA priority pollutant with national water quality standards, National Pollutant Discharge Elimination System (NPDES) permits or state/territory/tribe-specific equivalent permits are required for treatment in Waters of the United States. There also may be location-specific requirements on use or nonuse.
- Care is required when treating algae or cyanobacteria in soft waters due to the sensitivities of off-target species; copper algaecides can cause toxicity to some fish and invertebrates under certain conditions.
- Frequent application can lead to copper accumulation in sediments and potential adverse effects.
Copper algaecides have been applied in water bodies across the United States when problematic algae and cyanobacteria interfere with critical water resource uses and have mitigated nuisance bloom impacts to designated uses. Sites where copper algaecides have been effective range widely in terms of designated water resource uses, problematic algae or cyanobacteria, size (small ponds to thousands of hectare reservoirs), and trophic status. Copper algaecide applications can be scaled to the appropriate size for the water body but should be staged to accommodate potential significant declines in dissolved oxygen.
There are a wide range of copper algaecides on the market. Many are designed to specifically eliminate a target group of algae, cyanobacteria, or aquatic plants. While copper is a micronutrient and naturally present in many waters, copper applications can affect aquatic life, including fish and macroinvertebrates, under certain conditions (USEPA 1984). Copper may also accumulate in the bottom sediments over time (Hanson and Stefan 1984, Paul, Cruz-Rivera, and Thacker 2001), where there is potential for direct interaction with benthic organisms or for the copper to become solubilized into the water (Hanson and Stefan 1984, MacDonald, Ingersoll, and Berger 2000). It is important to follow the manufacturer’s label. You should also have a good understanding of your local water and sediment chemistry, as well as any previous cyanobacteria control efforts, when considering the use of copper algaecides. Pre- and post-application monitoring should be part of your management plan to assist you with evaluation of treatment success and potential adverse effects.
COST ANALYSIS
Relative cost per growing season: Copper algaecides
| ITEM | RELATIVE COST PER GROWING SEASON |
| Material | $ |
| Personal Protective Equipment | $$ |
| Equipment | $$ |
| Machinery | $ |
| Tools | $ |
| Labor | $$ |
| OVERALL | $ |
The cost of a treatment is a function of the area treated, labor, product used, and severity of the problematic algal or cyanobacterial issue. One previous study in New York had a $933/acre treatment cost.
CASE STUDY EXAMPLES
Hartwell Lake, Anderson, South Carolina, United States: A chelated copper algaecide and a peroxide compound was used to treat problematic algae, cyanobacteria, and their resulting taste and odor compounds, which had generated customer complaints associated with drinking water sourced from Hartwell Lake.
An adaptive water resource management approach was used to develop an effective treatment plan at the site. The approach included identification of the source of taste and odor compounds and small-scale laboratory studies to determine which algaecide should be used for treatment. A pilot treatment was applied initially, followed by three full-scale treatments during the growth season of the problematic species.
The adaptive water resource management approach and chelated copper algaecide and peroxide treatments eliminated customer complaints. This approach also resulted in a 50% cost savings relative to the previous year, when powder-activated carbon was used in-plant to manage taste and odor in potable water.
More information on this case study can be found in Huddleston et al. (2016).
REGULATORY AND POLICY CONSIDERATIONS
Copper algaecides require NPDES permits and are also regulated under the Federal Insecticide, Fungicide, and Rodenticide Act. States may have additional restrictions for water bodies with specific uses and require state permits to apply copper-based treatments. The use of a certified pesticide applicator or lake management company is often required. Post-application monitoring may also be required.
REFERENCES
Bishop, W. M., and J. H. Rodgers. 2011. “Responses of Lyngbya magnifica gardner to an algaecide exposure in the laboratory and field.” Ecotoxicol Environ Saf 74 (7):1832-8. doi: 10.1016/j.ecoenv.2011.06.007.
Calomeni, Alyssa J, Tyler D Geer, Kyla J Iwinksi, John H Rodgers, Jr., John D Madsen, and Ryan M Wersal. 2017. “Monitoring for National Pollutant Discharge Elimination System permit requirements: algaecides.” Journal of Integrated Pest Management 8 (1). doi: 10.1093/jipm/pmx025.
Calomeni, Alyssa J., Ciera M. Kinley, Tyler D. Geer, Maas Hendrikse, and John H. Rodgers. 2018. “Lyngbya wollei responses to copper algaecide exposures predicted using a concentration – exposure time ( CET ) model : influence of initial biomass.” J. Aquat. Plant Manage. 56:73-83.
Calomeni, Alyssa, John Rodgers, and Ciera Kinley-Baird. 2014. “Responses of Planktothrix agardhii and Pseudokirchneriella subcapitata to copper sulfate (CuSO4 · 5H2O) and a chelated copper compound (Cutrine®-Ultra).” Water Air and Soil Pollution 225:1-15. doi: 10.1007/s11270-014-2231-3.
Fitzgerald, G. P., and S. L. Faust. 1963. “Factors affecting the algicidal and algistatic properties of copper.” Applied microbiology 11 (4):345-351.
Gibson, C.E. . 1972. “The algicidal effects of copper on a green and a bluegreen alga and some ecological implications.” J. Appl. Ecol. 9:513-518.
Hanson, Mark J., and Heinz G. Stefan. 1984. “Side effects of 58 years of copper sulfate treatment of the Fairmont Lakes, Minnesota.” JAWRA Journal of the American Water Resources Association 20 (6):889-900. doi: 10.1111/j.1752-1688.1984.tb04797.x.
Huddleston, M. H., John H. Jr. Rodgers, Kalya Wardlaw, Tyler D. Geer, Alyssa J. Calomeni, Scott Willett, Jennifer J Barrington, David W. Melton, John P. Chastain, Martín Bowen, Mike Spacil, and SynTerra. 2016. “Adaptive Water Resource Management for Taste and Odor Control for the Anderson Regional Joint Water System.” https://www.synterracorp.com/taste-and-odor-control-in-source-water-for-the-anderson-regional-joint-water-system/.
Isaacs, David A., Russell G. Brown, William A. Ratajczyk, Nathan W. Long, John H. Rodgers Jr., and James C. Schmidt. 2013. “Solve taste-and-odor problems with customized treatment.” Opflow 39 (7):26-29. doi: 10.5991/opf.2013.39.0039.
Iwinski, K. J., J. H. Rodgers, Jr., C. M. Kinley, M. Hendrikse, A. J. Calomeni, A. D. McQueen, T. D. Geer, J. Liang, V. Friesen, and M. Haakensen. 2017. “Influence of CuSO(4) and chelated copper algaecide exposures on biodegradation of microcystin-LR.” Chemosphere 174:538-544. doi: 10.1016/j.chemosphere.2017.01.079.
Iwinski, Kyla, Andrew McQueen, Ciera Kinley-Baird, Alyssa Calomeni, Tyler Geer, and John Rodgers. 2016. “Sediment copper concentrations, in-situ benthic invertebrate abundance, and sediment toxicity: comparison of treated and untreated coves in a Southern reservoir.” Water, Air, & Soil Pollution 227. doi: 10.1007/s11270-016-2778-2.
Kinley, C. M., K. J. Iwinski, M. Hendrikse, T. D. Geer, and J. H. Rodgers, Jr. 2017. “Cell density dependence of Microcystis aeruginosa responses to copper algaecide concentrations: Implications for microcystin-LR release.” Ecotoxicol Environ Saf 145:591-596. doi: 10.1016/j.ecoenv.2017.08.010.
MacDonald, D. D., C. G. Ingersoll, and T. A. Berger. 2000. “Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems.” Archives of Environmental Contamination and Toxicology 39 (1):20-31. doi: 10.1007/s002440010075.
Moore, G. T., and K. F. Kellerman. 1905. “Copper as an Algicide and Disinfectant in Water Supplies.” U. S. Government Printing Office.
Murray-Gulde, C. L., J. E. Heatley, A. L. Schwartzman, and Jr J. H. Rodgers. 2002. “Algicidal effectiveness of Clearigate, Cutrine-Plus, and copper sulfate and margins of safety associated with their use.” Archives of Environmental Contamination and Toxicology 43 (1):19-27. doi: 10.1007/s00244-002-1135-1.
Paul, V.J., E. Cruz-Rivera, and R.W. Thacker. 2001. “Chemical mediation of macroalgal-herbivore interactions: ecological and evolutionary perspectives.” In Marine chemical ecology. CRC Marine Science Series, edited by J.B. McClintock and B.J Baker, 227-265. Boca Raton: CRC Press:.
USEPA. 1984. “Ambient Water Quality Criteria for Copper – 1984. EPA 440/5-84-031.” Washington, D, C https://www.epa.gov/sites/production/files/2019-03/documents/ambient-wqc-copper-1984.pdf.
DREDGING
In-lake Prevention Strategy
Limited Supporting Field Data
Dredging is the physical removal of sediment from the bottom of a water body. In the context of HCB control, dredging is performed to reduce the supply of nutrients from the sediment (internal loading) to the water column. There are many different dredging techniques available, but most can be categorized as either hydraulic or mechanical dredging. Hydraulic dredging works by sucking sediment through a tube to a barge or offshore location. Mechanical dredging involves excavating the sediment with backhoes, clamshells, draglines, or equipment. Dredging for HCB control purposes usually targets the upper, nutrient-enriched sediment layer (for example, 10–100 centimeters). The amount of dredged material to be removed can be determined by measuring phosphorus concentrations at varying depths in bottom sediments.
EFFECTIVENESS
- Water body type: Pond, lake/reservoir
- Depth: Cost may limit this technique to shallower water bodies
- Surface area: Cost may limit this technique to smaller water bodies
- Any trophic state
- Any mixing regime
- Any water body use
- Water bodies with high internal nutrient loading from sediments but controlled external loads
NATURE OF HCB
- Repeating or persistent HCBs
- Prevention strategy
Dredging can be a useful technique for water bodies that have experienced historical nutrient over-enrichment such that internal recycling of nutrients from the sediment is sufficient to support blooms (Peterson 1982). It has the highest potential for success in lakes where sediment fluxes are the dominant nutrient loading source and external nutrient loads have been controlled (Bormans, Marsálek, and Jancula 2015). Dredging also has been successful when combined with other control techniques, such as sediment phosphorus inactivation (Lürling and Faassen 2012).
ADVANTAGES
- Reduces internal nutrient loads
- Increases lake depth
- Dredge material can sometimes be beneficially reused
LIMITATIONS
- Not effective if external loads remain high
- Requires disposal of dredged material
- Requires permitting under the Clean Water Act (33 U.S.C. §§1251–1387, Sections 401 and 404)
- Temporarily increases turbidity
- Impacts bottom-dwelling aquatic life
- From cost perspective, usually applied in shallower systems

Figure C-3. Mechanical dredging on the upper Hudson River (A) and hydraulic dredging equipment on Easter Lake, IA (B).
Sources: USEPA (A) and Snyder and Associates (B). Used with permission.
CASE STUDY EXAMPLES
Lake Trummen, Sweden: Extensive efforts were made to control external nutrient loads to the historically polluted Lake Trummen in Sweden. After hydraulic dredging, cyanobacterial blooms largely disappeared and were replaced by a taxonomically rich phytoplankton community, and the recreational potential of the lake was greatly improved (Björk, Pokorný, and Hauser 2011).
Lake Vajgar, Czech Republic: An automatically controlled precision dredger was used to remove the top sediment layer in an attempt to reduce recurring HCBs. Although sediment nutrient fluxes decreased dramatically, external nutrient loading was still high, and HCBs continued to occur (Björk, Pokorný, and Hauser 2011).
The scientific literature shows that dredging has mixed results as an HCB control technique, depending upon whether external loads have also been controlled and whether nutrient limitations on algae can be imposed. See Bormans, Marsálek, and Jancula (2015) for a more extensive literature review of dredging as an HCB control technique.
COST ANALYSIS
Dredging is one of the most expensive HCB control techniques (Bormans, Marsálek, and Jancula 2015, Hudson 1998, Peterson 1982). The cost can vary greatly based on the area, depth, and nature of the material to be dredged. Pre-dredging costs typically include bathymetric surveys, permitting, and chemical analysis of the material to be dredged. Disposal costs are higher if the material is contaminated or if the disposal area is far from the lake or reservoir. Conversely, disposal costs can be lower if the material can be beneficially reused (for example, applied to pasture or crops as a soil amendment). The Illinois Environmental Protection Agency (Hudson 1998) estimated that typical costs of dredging in 2020 dollars are $8–$24 per cubic yard for hydraulic dredging and $13–$48 per cubic yard for mechanical dredging. For other examples, the average cost was $63,443/acre. Although is it possible to dredge water bodies of various size, costs may limit dredging’s practical use for HCB control to relatively small or shallow water bodies.
Relative cost per growing season: Dredging
| ITEM | RELATIVE COST PER GROWING SEASON |
| Planning/Permitting | $$ |
| Material | $ |
| Equipment | $$$ |
| Labor | $$$ |
| Disposal | $$ |
| OVERALL | $$$ |
REGULATORY AND POLICY CONSIDERATIONS
In the United States, Sections 401 and 404 of the Clean Water Act (33 U.S.C. §§1251–1387) require that those persons or businesses that propose dredging within navigable waters obtain a permit from the U.S. Army Corps of Engineers, the state regulatory agency, and (in some cases) USEPA. Permitting requirements can be streamlined somewhat by joint permit applications to multiple agencies. Typical permit application requirements include the quantity or extent of dredging, disposal location and method, and expected environmental impacts. In some cases, testing of the dredged material will be required, which could affect disposal requirements.
Dredging can have potential co-benefits of increased lake volume, enhanced boat navigation, removal of nuisance macrophytes, enhanced fish production, and removal of toxic sediments (Peterson 1982). In fact, most dredging projects are motivated by one or more of these drivers rather than by HCB control. In many settings, the level of stakeholder support for dredging projects will be tied to these co-benefits.
REFERENCES
Björk, Sven, Jan Pokorný, and Václav Hauser. 2011. “Restoration of lakes through sediment removal, with case studies from Lakes Trummen, Sweden and Vajgar, Czech Republic.” In Restoration of Lakes, Streams, Floodplains, and Bogs in Europe, edited by Martina Eiseltová, 101-122. Netherlands: Springer
Bormans, Myriam, Blahoslav Marsálek, and Daniel Jancula. 2015. “Controlling internal phosphorus loading in lakes by physical methods to reduce cyanobacterial blooms: a review.” Aquatic Ecology 50. doi: 10.1007/s10452-015-9564-x.
Hudson, Holly. 1998. “Lake dredging.” Lake Notes. doi: https://epa.illinois.gov/content/dam/soi/en/web/epa/documents/water/conservation/lake-notes/lake-dredging.pdf.
Lürling, M., and E. J. Faassen. 2012. “Controlling toxic cyanobacteria: effects of dredging and phosphorus-binding clay on cyanobacteria and microcystins.” Water Res 46 (5):1447-59. doi: 10.1016/j.watres.2011.11.008.
Peterson, Spencer A. 1982. “Lake restoration by sediment removal.” Journal of the American Water Resources Association 18 (3):423-436. doi: 10.1111/j.1752-1688.1982.tb00009.x.
FLOATING WETLANDS
In-lake Prevention Strategy
Limited Supporting Data
Artificial floating vegetated islands have been employed since the 1970s and 1980s for removing nutrients from ponds, lakes, reservoirs, and brackish bays, thus discouraging the algal/cyanobacterial blooms that are favored by high nutrient levels (Hoeger 1988). Floating wetlands have been deployed many times in Asia (Lu, Ku, and Chang 2015, Ning et al. 2014). Islands of various sizes, materials, and designs have been constructed to provide platforms for suspending a variety of emergent plant vegetation. The vegetation is integral to the designs, because the plants take up dissolved nutrients through their roots, which are suspended in the aquatic ecosystem.
This “hydroponics-like” approach for nutrient capture is conceptually and theoretically sound (Wang and Sample 2013). Plant roots suspended in the water below the islands continually sequester nutrients from the water. Development of microbial communities attached to the roots of the plants further increases the drawdown of dissolved nutrients (Masters 2012). Periodic harvesting of plant material from the island results in a net removal of nutrients from the system.
EFFECTIVENESS
- Water body type: Pond, lake/reservoir
- Surface area: Small
- Depth: Shallow
- Trophic state: Eutrophic, hypereutrophic
- Any mixing regime
- Any water body use
NATURE OF HCB
- All HCB types
- Singular or repeating HCBs
- Toxic and nontoxic HCBs
- Targets all algal species
- Prevention strategy
The effectiveness of this approach for nutrient reduction is determined by a number of factors, and the field is still optimizing the approach (Dunqiu et al. 2012). Ultimately, this technique’s effectiveness depends on the surface area covered by islands relative to the volume of the water body, as well as the magnitude of internal and external nutrient loading relative to the rate of nutrient removal by the islands. The rate of nutrient capture and removal by these islands is dependent not only on island size but also on the type of vegetation employed and environmental factors that affect primary production by the plants (for example, light, temperature, and nutrient concentrations in the water body). These features are difficult to quantify, making it difficult to create a generalized approach that will be universally successful. The Chesapeake Bay Program convened two expert panels to set nutrient removal efficiencies and concluded that these features were appropriate for stormwater ponds and not open waters, where 10%–50% aerial coverage would result in increasing nutrient removal credit toward Total Maximum Daily Load (TMDL) limits (Schueler, Lane, and Wood 2016).
A number of anecdotal reports or one-off success stories have claimed effectiveness, but relatively few scientific studies have clearly demonstrated that the approach can result in significant nutrient reduction (Geng et al. 2017, Lu, Ku, and Chang 2015, Vázquez-Burney et al. 2015). Successes are also reported in non-peer-reviewed fact sheets and reports, as well as abstracts from conference proceedings or other documents and publications that have not been peer-reviewed. Growth and nutrient uptake by several candidate plant species have been tested on artificial islands (Geng et al. 2017, Yao et al. 2011, Zhu, Li, and Ketola 2011), demonstrating that nutrients are indeed acquired by the island plants. However, studies demonstrating that such nutrient uptake and removal is a significant fraction of the total nutrient load of the aquatic ecosystem are scarce.
The anticipated or supposed effect of floating islands is a reduction in nutrient concentrations, resulting in overall reduction of algal/cyanobacterial growth. However, it is also possible (but largely untested) that shifts in plankton community structure away from harmful or noxious algal/cyanobacterial species may occur due to the activities of the plants or their attendant root microbes. In addition, reductions or changes in the plankton community as a consequence of reduced light penetration (due to the presence of the floating islands) have been suggested but not quantified.
ADVANTAGES
- Reduced nutrient loads by uptake and removal of plant tissue
- Reduced light penetration into the water column, reducing primary production
- Reduced wind-driven circulation may reduce deep mixing of the water column, reducing nutrient transport
- Thermal insulation may reduce high water temperatures in the summer, constraining cyanobacterial growth
- Plant roots provide increased biotic surface area for microbial growth, enhancing nutrient removal
- Plant root microbes may increase predation on the planktonic microbes
- Application has been carried out in freshwater and brackish environments
- Can be harvested
LIMITATIONS
- High cost of island design, construction, deployment, maintenance, harvesting, and replanting
- Substantive nutrient reduction in the treated water body requires prolonged use
- Rate of nutrient removal must be high relative to internal loads and greater than external loading
- Plant growth is most rapid and luxurious at high (hypereutrophic) nutrient concentrations (Cao and Zhang 2014); efficacy at “environmentally relevant” concentrations is not clear
- Reduced wind-driven circulation may reduce deep mixing of the water column and lead to greater stratification and increases in nutrients from low-oxygen bottom sediments
CASE STUDY EXAMPLES
Florida, United States: Floating wetland islands resulted in a 32% removal of nitrogen (mostly organic nitrogen) in the outflow from a reservoir receiving wastewater effluent (Vázquez-Burney et al. 2015).
Laboratory-scale: Stewart et al. (2008) conducted a test of nitrogen and phosphorus removal from simulated agricultural and wastewater runoff (liquid hydroponic fertilizer). Removal of both elements was demonstrable, at least at very high dissolved nutrient concentrations. Such results are proof-of-concept for artificial islands, but there is still very limited information on how effective the approach will be at much lower, environmentally relevant nutrient concentrations (Stewart et al. 2008).
Stormwater retention ponds, Auckland, New Zealand: (Borne 2014) examined phosphorus removal by floating wetlands on water passing through stormwater retention ponds. Results indicated that the floating wetlands reduced phosphorus in the water discharged from the ponds, but sedimentation, rather than uptake by plants, was the main process reducing phosphorus in the discharge water (Borne 2014).
COST ANALYSIS
There are significant costs associated with the deployment and maintenance of floating islands. Commercial entities offer design, construction, deployment, and maintenance services. Islands require substantial materials and labor for their construction, mooring, and planting. Maintaining, harvesting, and replanting islands can be labor intensive, while removal (which may be necessary seasonally) and redeployment can be costly. Harvested plant materials must also be composted or otherwise removed. Finally, environmental monitoring should be conducted (for example, water clarity, nutrient concentrations, phytoplankton characterizations) to document that the artificial islands are positively affecting water quality and reducing nutrient loads within the water body.
Relative cost per growing season: Floating wetlands
| ITEM | RELATIVE COST PER GROWING SEASON |
| Material | $$$ |
| Personal Protective Equipment | $ |
| Equipment | $ |
| Machinery | $ |
| Tools | $ |
| Labor | $$$ |
| O&M Costs | $$ |
| OVERALL | $$$ |
REGULATORY AND POLICY CONSIDERATIONS
Permits for deployment of artificial islands vary widely depending on ownership, management, and jurisdiction of the water body. For example, private lakes may require only permission from the homeowner’s association, whereas fresh and brackish waters under municipal, state, or federal jurisdiction may involve permitting from the city, county, state, or federal government (for example, USACE).
Public acceptance of this approach stems largely from public perspective on the islands themselves, and that is typically—and often decidedly—mixed. Some residents accept the islands (if they are well designed and deployed), as they provide clear evidence that “something is being done” to address an existing problem. However, aesthetics are important to all users of the water body. Complaints about artificial islands as “eyesores” are not unusual among neighbors and visitors. More significantly, hindrances to boating, water skiing, and other recreational activities are potential detractors for the public.
REFERENCES
Borne, Karine E. 2014. “Floating treatment wetland influences on the fate and removal performance of phosphorus in stormwater retention ponds.” Ecological Engineering 69:76-82. doi: https://doi.org/10.1016/j.ecoleng.2014.03.062.
Cao, Wenping, and Yanqiu Zhang. 2014. “Removal of nitrogen (N) from hypereutrophic waters by ecological floating beds (EFBs) with various substrates.” Ecological Engineering 62:148–152. doi: 10.1016/j.ecoleng.2013.10.018.
Dunqiu, W., B. Shaoyuan, W. Mingyu, X. Qinglin, Z. Yinian, and Z. Hua. 2012. “Effect of artificial aeration, temperature, and structure on nutrient removal in constructed floating islands.” Water Environ Res 84 (5):405-10. doi: 10.2175/106143012×13347678384684.
Geng, Yan, Wenjuan Han, Chenchen Yu, Qinsu Jiang, Jianzhi Wu, Jie Chang, and Ying Ge. 2017. “Effect of plant diversity on phosphorus removal in hydroponic microcosms simulating floating constructed wetlands.” Ecological Engineering 107:110-119. doi: 10.1016/j.ecoleng.2017.06.061.
Hoeger, Sven. 1988. “Schwimmkampen.” Germany’s artificial floating islands 43 (4):304-306.
Lu, Hsiao-Ling, Chen-Ruei Ku, and Yuan-Hsiou Chang. 2015. “Water quality improvement with artificial floating islands.” Ecological Engineering 74:371-375. doi: 10.1016/j.ecoleng.2014.11.013.
Masters, Bernie. 2012. “The ability of vegetated floating Islands to improve water quality in natural and constructed wetlands: a review.” Water Practice and Technology 7 (1). doi: 10.2166/wpt.2012.022.
Ning, D., Y. Huang, R. Pan, F. Wang, and H. Wang. 2014. “Effect of eco-remediation using planted floating bed system on nutrients and heavy metals in urban river water and sediment: a field study in China.” Sci Total Environ 485-486:596-603. doi: 10.1016/j.scitotenv.2014.03.103.
Schueler, Tom, Cecilia Lane, and David Wood. 2016. “Recommendations of the Expert Panel to Define Removal Rates for Floating Treatment Wetlands in Existing Wet Ponds. July 2016.” https://www.chesapeakebay.net/documents/FINAL-FTW-EXPERT-PANEL-REPORT-072716-LONG.pdf.
Stewart, Frank, Tim Mulholland, Alfred Cunningham, Bruce Kania, and Mark Osterlund. 2008. “Floating islands as an alternative to constructed wetlands for treatment of excess nutrients from agricultural and municipal wastes – results of laboratory-scale tests.” Land Contamination & Reclamation 16:25-33. doi: 10.2462/09670513.874.
Vázquez-Burney, R., J. Bays, R. Messer, and J. Harris. 2015. “Floating wetland islands as a method of nitrogen mass reduction: results of a 1 year test.” Water Sci Technol 72 (5):704-10. doi: 10.2166/wst.2015.235.
Wang, Chih-Yu, and David J. Sample. 2013. “Assessing floating treatment wetlands nutrient removal performance through a first order kinetics model and statistical inference.” Ecological Engineering 61:292-302. doi: 10.1016/j.ecoleng.2013.09.019.
Yao, K. , S. Song, Zhang, Z. Xu , Zhang, J. Liu, L. Cheng, and J. Liu. 2011. “Vegetation characteristics and water purification by artificial floating island ” African Journal of Biotechnology 10 (82):19119-19125. doi: 10.5897/AJB11.2964.
Zhu, Liandong, Zhaohua Li, and Tarja Ketola. 2011. “Biomass accumulations and nutrient uptake of plants cultivated on artificial floating beds in China’s rural area.” Ecological Engineering 37 (10):1460-1466. doi: 10.1016/j.ecoleng.2011.03.010.
FOOD WEB MANIPULATION
In-lake Intervention and Prevention Strategy
Substantial Supporting Field Data
Manipulating fish populations in ponds, lakes, and reservoirs to control cyanobacteria populations has been undertaken in multiple locations throughout the world over the past four to five decades. Some investigations report successful reduction of cyanobacteria biomass through stocking of herbivorous fish populations that ingest cyanobacteria, such as silver and bighead carp (Xie and Liu 2001). Zhang, Xie, and Huang (2008) suggest that stocking with filter feeders (carp) in lakes with low macrozooplankton densities will reduce phytoplankton and cyanobacteria, as cyanobacteria have been shown to make up 84.4% of the phytoplankton silver carp consume (Chen et al. 2006). Other investigations remove fishes that graze on zooplankton (Pot and Heerdt 2014), while still others increase piscivorous fish stocks (Carpenter, Kitchell, and Hodgson 1985) to increase large fish predation on planktivorous fish that ingest herbivorous zooplankton, such as Daphnids; by doing so, the herbivorous zooplankton can increase to consume developing cyanobacteria.
Recent reviews (Lürling and Mucci 2020, Triest, Stiers, and Van Onsem 2016) suggest substantial uncertainty with these approaches and propose that combining these techniques with other strategies or simply using other options may offer better chances for success at reducing cyanobacteria. The former research group summarized data from 34 studies that employed stocking with herbivorous fishes, fish removal, or stocking with piscivores. Adding filter feeding fishes succeeded in 4 of 6 times in reducing lake cyanobacteria; fish removal was successful in 6 of 8 projects, while the addition of piscivores was successful only 2 of 5 instances. When fish removal and piscivore stocking were combined, cyanobacteria declined in five lakes. Manipulation of fish through removal or piscivore additions, when combined with one or multiple additional strategies, was successful 5 of 6 times. These authors state, “Reasons for success or failure … could be explained through bottlenecks encountered with fish removal, stocking densities, cascading effects, associated zooplankton grazing, diet shifts away from cyanobacteria, macrophyte recovery, nutrient or pH status.”
Hence, results from manipulating higher trophic levels of a water body’s food web remain uncertain and unpredictable.
EFFECTIVENESS
- Highly variable results
- Any water body type
- Any surface area or depth
- Any trophic state, but typically most effective in eutrophic systems
- Mixing regime: Meromictic, monomictic, or dimictic
- Any water body use
NATURE OF HCB
- Many HCB species
- Toxic and nontoxic HCBs
- Intervention and prevention strategy
Food web manipulations require substantial short- and long-term monitoring prior to and following treatment, not only for cyanobacteria but also for densities of fish species and crustacean zooplankton. Adjustments in fish stocks may be necessary over time, necessitating a substantial investment in time and money. In addition, nutrient concentrations and turbidity should also be monitored, as adding fishes can induce bottom disturbance, nutrient release, and sediment resuspension.
ADVANTAGES
- Elimination of HCBs in some systems
- Reported improved water quality, clarity, and ecological benefits in some cases
LIMITATIONS
- Highly variable results
- Substantial costs
- Some cyanobacteria survive fish gut passage to “seed” blooms in future years
- Requires water quality, plankton, and fish monitoring pretreatment and short- and long-term (yearly) thereafter
- Fish stock estimates are often uncertain
- May require yearly adjustments in fish stocks
COST ANALYSIS
Relative cost per growing season: Food web manipulation
| ITEM | RELATIVE COST PER GROWING SEASON |
| Material | $$$ |
| Personal Protective Equipment | $ |
| Equipment | $$ |
| Machinery | $$ |
| Labor | $$ |
| O&M Costs | $$ |
| OVERALL | $$ |
CASE STUDY EXAMPLES
Netherlands: ter Heerdt and Hootsmans (2007) report an 85-ha shallow peaty lake fish removal that resulted in <25 kg/ha benthivorous fish and <15 kg/ha planktivorous fish stocks. This removal resulted in clear water, reduced filamentous cyanobacteria, and increased Bosmina spp. populations. Following cyanobacteria disappearance, Daphnia spp. dominated the zooplankton that kept phytoplankton abundances low.
China: Lu et al. (2006) Lu et al. (2006) stocked Lake Yuehu with herbivorous tilapia (Oreochromis niloticus) at 3–5 g/m3. Compared to the previous year’s 70% cyanobacteria, the cyanobacteria biomass was reduced to 22.1% in 2001 and 11.2% in 2002. In another system, tilapia fingerlings were added at 8–15 g/m3. The cyanobacteria bloom disappeared in 20 days.
Texas, United States: In contrast to the successes above, largemouth bass were stocked in a Texas reservoir. Although the impact passed down to the phytoplankton, cyanobacteria densities did not change, and large cyanobacteria replaced edible phytoplankton species (Drenner et al. 2002).
REGULATORY AND POLICY CONSIDERATIONS
State officials should be consulted on any plans to remove or add fish stocks to natural waters.
REFERENCES
Carpenter, Stephen R., James F. Kitchell, and James R. Hodgson. 1985. “Cascading trophic interactions and lake productivity.” BioScience 35 (10):634-639. doi: 10.2307/1309989.
Chen, Jun, Ping Xie, Dawen Zhang, Zhixin Ke, and Hua Yang. 2006. “In situ studies on the bioaccumulation of microcystins in the phytoplanktivorous silver carp (Hypophthalmichthys molitrix) stocked in Lake Taihu with dense toxic Microcystis blooms.” Aquaculture 261 (3):1026-1038. doi: 10.1016/j.aquaculture.2006.08.028.
Drenner, Ray W. , Robert M. Baca, Jeffrey S. Gilroy , Mark R. Ernst, David J. Jensen , and David H. Marshall. 2002. “Community responses to piscivorous largemouth bass: a biomanipulation experiment.” Lake and Reservoir Management 18 (1):44-51. doi: 10.1080/07438140209353928.
Lu, Kaihong, Chunhua Jin, Shuanglin Dong, Binhe Gu, and Stephen H. Bowen. 2006. “Feeding and control of blue-green algal blooms by tilapia (Oreochromis niloticus).” Hydrobiologia 568 (1):111-120. doi: 10.1007/s10750-006-0023-5.
Lürling, Miquel, and Maíra Mucci. 2020. “Mitigating eutrophication nuisance: in-lake measures are becoming inevitable in eutrophic waters in the Netherlands.” Hydrobiologia. doi: 10.1007/s10750-020-04297-9.
Pot, Roelf, and Gerard Heerdt. 2014. “Succession dynamics of aquatic lake vegetation after restoration measures: increased stability after 6 years of development.” Hydrobiologia 737:333-45. doi: 10.1007/s10750-014-1835-3.
ter Heerdt, Gerard, and Michiel Hootsmans. 2007. “Why biomanipulation can be effective in peaty lakes.” Hydrobiologia 584 (1):305-316. doi: 10.1007/s10750-007-0594-9.
Triest, Ludwig, Iris Stiers, and Stijn Van Onsem. 2016. “Biomanipulation as a nature-based solution to reduce cyanobacterial blooms.” Aquatic Ecology 50 (3):461-483. doi: 10.1007/s10452-015-9548-x.
Xie, Ping, and Jiankang Liu. 2001. “Practical success of biomanipulation using filter-feeding fish to control cyanobacteria blooms: A synthesis of decades of research and application in a subtropical hypereutrophic lake.” The Scientific World Journal 1:337-56. doi: 10.1100/tsw.2001.67.
Zhang, X., P. Xie, and X. Huang. 2008. “A review of nontraditional biomanipulation.” ScientificWorldJournal 8:1184-96. doi: 10.1100/tsw.2008.144.
HYDRAULIC FLUSHING
In-lake Intervention and Prevention Strategy
Substantial Supporting Data
It is well established that vertical water column stability and long water residence times favor cyanobacteria over eukaryotic phytoplankton (Ibelings et al. 2016, Mitrovic et al. 2003, Paerl et al. 2016). Thus, the disruption of these conditions can, under certain circumstances, reduce nuisance HCBs (Havens et al. 2019, Lehman 2014, McDonald and Lehman 2013). Management strategies that change the hydraulics by flushing (shorter water retention time) can be effective management tools that both affect nutrient delivery to HCBs and disrupt habitat conditions that favor HCB development (calm, warm water) in smaller water bodies (Paerl et al. 2016). The geographic setting of the water body and lake depth will dictate which type of in-lake management strategy is feasible, based on water availability or lack thereof. For example, arid western regions of the United States may have more restrictions than eastern to midwestern regions.
In-lake hydraulics may be defined as the movement of water such as surface waves or internal waves that are influenced by wind mixing, internal currents influenced by tributary inflows or discharge, stratified water layers influenced by density gradients, or concentrations that affect turbulent mixing within the water body (Starosolszky 1974). Disrupting seasonal stratification by changing reservoir hydraulics can promote the development of diatom and green algae rather than cyanobacteria.
Lake and reservoir flushing may be defined as the passthrough of a large volume of water, preferably lower in nutrient concentrations, with sufficient velocity to flush lake water containing cyanobacteria downstream before cyanobacteria populations can regrow in the water body (Ibelings et al. 2016, Mitrovic, Hardwick, and Dorani 2010). Flushing reduces the water retention time (Romo et al. 2012) and disrupts water column stability, thereby minimizing the contact time between cyanobacteria and nutrients while eliminating calm waters that favor growth of buoyant cyanobacteria species (Anderson, Komor, and Ikehata 2014). Reservoir flushing may also be defined as the seasonal release of hypolimnetic water from thermally stratified lakes that are enriched with bioavailable nutrients from internal nutrient loading (Nürnberg 2007). The discharge of water before fall turnover reduces the amount of nutrient-rich hypolimnetic water that mixes with near-surface epilimnetic water and may reduce cyanobacteria blooms that occur post-turnover.
The frequency of flushing flows may also affect the proliferation of benthic cyanobacterial mats (Quiblier et al. 2013). Wood, Wagenhoff, and Young (2014) estimated the specific flushing flows necessary to reduce Phormidium cover below 20% for multiple locations in New Zealand rivers. A study across multiple New Zealand river systems demonstrated accrual of this cyanobacterium also increased with time since the last flushing flow (McAllister et al. 2018). Stanfield (2018) derived river discharge thresholds that, once exceeded, removed attached benthic cyanobacteria in the upper Potomac River in Maryland.
EFFECTIVENESS
- Water body types: Lake/reservoir
- Any surface area
- Depth: Shallow
- Trophic state: Eutrophic
- Mixing strategy: Polymictic
- Water body uses: Recreation, drinking water source
- Requires more planning for water management
- Reservoir releases of 80 MGD (critical flow velocity of 1 foot/second) have been effective in mitigating HCB development via suppression of stratification and cell washout
- Reservoir releases of 800 MGD have been effective in removing an established HCB
- Run-of-river reservoirs are more suitable for managing hydraulics given flow conditions
NATURE OF HCB
- Effective on most types of cyanobacteria in the epilimnion
- Microcystis colonies in sheltered inlets or bays may be less affected by flushing
- Large releases of 80 MGD were effective in suppressing Anabaena circinalis
- In stratified lakes, flushing may not affect cyanobacteria in the metalimnion
- Flushing flows may reduce accrual of benthic cyanobacterial mats in rivers
- Delay timing of occurrent for nitrogen-fixing (Aphanizomenon) and non-nitrogen-fixing taxa (Microcystis)
- Change in algal composition favoring diatomsIntervention and prevention strategy
Flushing management strategies have been moderately effective in eutrophic lakes and reservoirs of less than 125 surface acres (Cross et al. 2014, James, Eakin, and Barko 2004, Pawlik-Skowronska and Toporowska 2016), as well as in some larger reservoirs, provided that sufficient flows are available (Qin et al. 2010). Releases of 80 MGD with a critical flow velocity of 1 foot/second have been effective in mitigating HCB development in a large reservoir by suppressing thermal stratification along with cell washout. Reservoir releases of 800 MGD have been effective in removing an established HCB (Lehman 2014).
ADVANTAGES
- Variability in regional rainfall patterns may benefit flushing capability, influence water residence time and stratification, and change cyanobacteria dominance and persistence
- Horizontal flushing by increasing the flowthrough of water can reduce HCB development via reduction in nutrient supply
- Does not require capital or equipment investment
- Weigh the cost of water versus intangible cost of closing water body due to HCBs
- A series of reservoirs may be managed to store and release water for the benefit of flushing a downstream reservoir
- Numerical modeling may indicate that changing reservoir hydraulics or flushing may or may not improve nutrient water quality or HCB conditions
- Short pulses of water spread out over the season may be as effective as one flushing event for planktonic species
LIMITATIONS
- Large volumes of low-nutrient water are needed to flush a reservoir
- Variable costs; can be low to expensive
- Not practical or effective on larger reservoirs
- Drinking water or irrigation reservoirs generally do not have the luxury of water surplus for flushing
- Requires more long-term planning to coordinate flushing events
- Changing reservoir hydraulics may warm the bottom water, affecting cold-water fisheries
- Potential for downstream impacts related to HCBs and cyanotoxins during flushing events
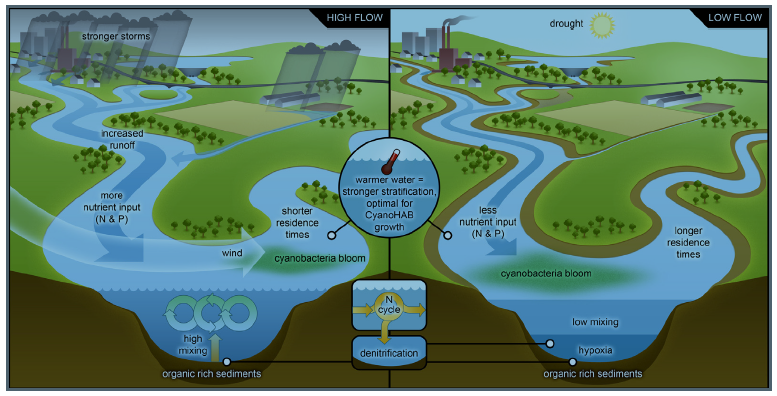
Figure C-5. The benefit of high-flow conditions on water column mixing, shorter retention times, and reduced HCB formation compared to low-flow conditions, longer retention times, and the persistence of thermal stratification, which all promote HCB formation.
Source: (Paerl et al. 2016). Used with permission.
Regional rainfall patterns may benefit flushing capability, influence water residence time, and change cyanobacteria dominance and persistence (Jagtman, Van der Molen, and Vermij 1992, Larsen et al. 2020). Other environmental factors—such as thermal stratification, water temperature, and potential fisheries—should be considered before implementing this strategy (Fulton III and Hendrickson 2011, Nelson et al. 2018). Often, numerical modeling can help evaluate these environmental factors and determine whether changing the reservoir hydraulics or flushing will be beneficial for the reservoir. The cost of raw water and limited supplies in many regions of the United States may also influence the decision to implement this lake management strategy. In these cases, the intangible cost (economics) of closing a water body due to HCBs should also be considered.
COST ANALYSIS
Relative cost per growing season: Hydraulic flushing
| ITEM | RELATIVE COST PER GROWING SEASON |
| Water Availability | $$–$$$ |
| O&M Costs | $–$$$ |
| OVERALL | $$–$$$ |
Financial costs depend on site-specific geographical settings and water availability. For example, if hydroelectric facilities are associated with run-of-the river facilities, the financial tradeoffs of water, electric power, and public perception must be thoroughly vetted before hydraulic, flushing, or drawdown management strategies are implemented. In the arid West, water availability and the cost of water severely limit the feasibility of hydraulic or flushing strategies, although water level drawdown may be more practical in this region.
Nearly all in-lake prevention and intervention techniques, including flushing and water level drawdown, will require some form of permitting or approval at the federal, state, or local level (Holdren, Jones, and Taggart 2001). Because these management strategies have the potential to flush sediment, nutrients, cyanobacteria, cyanotoxins, and other metalloid or hydrocarbon compounds to downstream regulated water bodies (as well as affect streamflow and water availability downstream), the state water quality regulatory office is the most appropriate agency to contact early in the planning phase.
Regulatory planning for hydraulic, flushing, and drawdown techniques may include but is not limited to Clean Water Act Sections 401 or 404 permitting, NPDES permitting, drawdown permitting, or Water Rights Administration permitting. Depending on the scale of the project and the extent of stakeholders, permitting could take months to years, so planning is critical. Depending on the size of the water body, its physical characteristics, and its environmental setting, implementing these techniques as short-term intervention approaches may require extensive planning.
REFERENCES
CASE STUDY EXAMPLES
Various locations: Bakker and Hilt (2016) identified 13 case studies that illustrate key factors to consider when implementing this management tool to reduce or prevent cyanobacteria blooms.
Lake Williston, Maryland, United States: Sellner et al. (2015) employed manually operated wooden dam control in a 67-acre lake to flush overwintering cyanobacteria populations from bottom sediments, thereby reducing potential “seed” populations for summer growth.
Anderson, Michael A., Andy Komor, and Keisuke Ikehata. 2014. “Flow routing with bottom withdrawal to improve water quality in Walnut Canyon Reservoir, California.” Lake and Reservoir Management 30 (2):131-142. doi: 10.1080/10402381.2014.898720.
Bakker, Elisabeth S., and Sabine Hilt. 2016. “Impact of water-level fluctuations on cyanobacterial blooms: options for management.” Aquatic Ecology 50 (3):485-498. doi: 10.1007/s10452-015-9556-x.
Cross, Iain, Suzanne McGowan, T. Needham, and C. Pointer. 2014. “The effects of hydrological extremes on former gravel pit lake ecology: management implications.” Fundamental and Applied Limnology 185. doi: 10.1127/fal/2014/0573.
Fulton III, R. S., and J. C. Hendrickson. 2011. Relationships of Water Flow with Plankton and Water Quality In St. Johns River Water Management District. Palatka, FL.
Havens, Karl E., Gaohua Ji, John R. Beaver, Rolland S. Fulton, and Catherine E. Teacher. 2019. “Dynamics of cyanobacteria blooms are linked to the hydrology of shallow Florida lakes and provide insight into possible impacts of climate change.” Hydrobiologia 829 (1):43-59. doi: 10.1007/s10750-017-3425-7.
Holdren, C. , W. Jones, and J. Taggart. 2001. “Managing Lakes and Reservoirs.” North American Lake Management Society; Terrene Insitute. https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=20004KKC.txt.
Ibelings, Bastiaan W., Myriam Bormans, Jutta Fastner, and Petra M. Visser. 2016. “CYANOCOST special issue on cyanobacterial blooms: synopsis—a critical review of the management options for their prevention, control and mitigation.” Aquatic Ecology 50 (3):595-605. doi: 10.1007/s10452-016-9596-x.
Jagtman, E., D.T. Van der Molen, and S Vermij. 1992. ” The influence of flushing on nutrient dynamics, composition and densities of algae and transparency in Veluwemeer, The Netherlands.” In Restoration and Recovery of Shallow Eutrophic Lake Ecosystems in The Netherlands. Developments in Hydrobiology, edited by L. Van Liere and R.D. Gulati. Dordrecht: Springer.
James, William F. , Harry L. Eakin, and John W. Barko. 2004. “Limnological Responses to Changes in the Withdrawal Zone of Eau Galle Reservoir, Wisconsin. ERDC WQTN-MS-07.” U. S. Army Engineer Research and Development Center. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.549.1531&rep=rep1&type=pdf.
Larsen, Megan L., Helen M. Baulch, Sherry L. Schiff, Dana F. Simon, Sébastien Sauvé, and Jason J. Venkiteswaran. 2020. “Extreme rainfall drives early onset cyanobacterial bloom.” bioRxiv:570275. doi: 10.1101/570275.
Lehman, John T. 2014. “Understanding the role of induced mixing for management of nuisance algal blooms in an urbanized reservoir.” Lake and Reservoir Management 30 (1):63-71. doi: 10.1080/10402381.2013.872739.
McAllister, Tara G., Susanna A. Wood, Javier Atalah, and Ian Hawes. 2018. “Spatiotemporal dynamics of Phormidium cover and anatoxin concentrations in eight New Zealand rivers with contrasting nutrient and flow regimes.” The Science of the total environment 612:71-80. doi: 10.1016/j.scitotenv.2017.08.085.
McDonald, Kahli E., and John T. Lehman. 2013. “Dynamics of Aphanizomenon and Microcystis (cyanobacteria) during experimental manipulation of an urban impoundment.” Lake and Reservoir Management 29 (2):103-115. doi: 10.1080/10402381.2013.800172.
Mitrovic, S. M., R. L. Oliver, C. Rees, L. C. Bowling, and R. T. Buckney. 2003. “Critical flow velocities for the growth and dominance of Anabaena circinalis in some turbid freshwater rivers.” Freshwater Biology 48 (1):164-174. doi: 10.1046/j.1365-2427.2003.00957.x.
Mitrovic, Simon M., Lorraine Hardwick, and Forugh Dorani. 2010. “Use of flow management to mitigate cyanobacterial blooms in the Lower Darling River, Australia.” Journal of Plankton Research 33 (2):229-241. doi: 10.1093/plankt/fbq094.
Nelson, Natalie G., Rafael Muñoz-Carpena, Edward J. Phlips, David Kaplan, Peter Sucsy, and John Hendrickson. 2018. “Revealing biotic and abiotic controls of harmful algal blooms in a shallow subtropical lake through statistical machine learning.” Environmental Science & Technology 52 (6):3527-3535. doi: 10.1021/acs.est.7b05884.
Nürnberg, Gertrud K. 2007. “Lake responses to long-term hypolimnetic withdrawal treatments.” Lake and Reservoir Management 23 (4):388-409. doi: 10.1080/07438140709354026.
Paerl, H. W., W. S. Gardner, K. E. Havens, A. R. Joyner, M. J. McCarthy, S. E. Newell, B. Qin, and J. T. Scott. 2016. “Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients.” Harmful Algae 54:213-222. doi: 10.1016/j.hal.2015.09.009.
Pawlik-Skowronska, Barbara, and Magdalena Toporowska. 2016. “How to mitigate cyanobacterial blooms and cyanotoxin production in eutrophic water reservoirs?” Hydrobiologia 778 (1):45-59. doi: 10.1007/s10750-016-2842-3.
Qin, B., G. Zhu, G. Gao, Y. Zhang, W. Li, H. W. Paerl, and W. W. Carmichael. 2010. “A drinking water crisis in Lake Taihu, China: linkage to climatic variability and lake management.” Environ Manage 45 (1):105-12. doi: 10.1007/s00267-009-9393-6.
Quiblier, Catherine, Susanna Wood, Isidora Echenique, Mark Heath, Villeneuve Aurelie, and Jean-François Humbert. 2013. “A review of current knowledge on toxic benthic freshwater cyanobacteria – Ecology, toxin production and risk management.” Water research 47. doi: 10.1016/j.watres.2013.06.042.
Romo, Susana, Juan Soria, Francisca Fernández, Youness Ouahid, and Ángel Barón-Solá. 2012. “Water residence time and the dynamics of toxic cyanobacteria.” Freshwater Biology 58 (3):513-522. doi: 10.1111/j.1365-2427.2012.02734.x.
Sellner, Kevin, Allen Place, Ernest Williams, Yonghui Gao, Elizabeth VanDolah, Michael Paolisso, Holly Bowers, and Shannon Roche. 2015. “Hydraulics and Barley Straw (Hordeum vulgare) as effective Treatment Options for a Cyanotoxin-Impacted Lake.” International Conference on Harmful Algae.
Stanfield, Kevin. 2018. “Developing methods to differentiate species and estimate coverage of benthic autotrophs in the potomac using digital imaging.” M. S. Thesis, Environmental Biology, Hood College.
Starosolszky, Ö. 1974. “Lake hydraulics.” Hydrological Sciences Bulletin 19 (1):99-114. doi: 10.1080/02626667409493874.
Wood, Susie, Annika Wagenhoff, and Roger Young. 2014. “The Effect of River Flow and Nutrients on Phormidium Abundance and Toxin Production in Rivers in the Manawatu-Whanganui Region.” Horizons Regional Council. https://envirolink.govt.nz/assets/Envirolink/Reports/1454-HZC108.pdf.
HYDRODYNAMIC CAVITATION
In-lake Intervention Strategy
Emerging Supporting Field Data
Hydrodynamic cavitation is a process to induce a phase change in water from a liquid to gas as microbubbles. There are a number of ways to produce these bubbles, including forcing water through a narrow constriction. As the bubbles collapse, a pressure (shock) wave is created that induces shear to disrupt colonies and aggregates. More importantly, bubble collapse yields hydroxyl and oxygen radicals, strong oxidizing agents that inhibit or kill cyanobacteria due to the group’s limited enzymatic capabilities to thwart strong oxidizing conditions. As in the response to ozonation and the emerging nanobubbling technology, cyanobacteria buoyancy control can be lost due to collapse of intracellular gas vesicles; disintegration of the membrane surrounding the cell is also possible through lipid peroxidation within the cell membrane (Li et al. 2015) but has not always been found (Jančula et al. 2014). Zhang et al. (2006) reported loss of photosynthesis due to destruction of chlorophyll and phycocyanin, thereby preventing subsequent growth. Laboratory studies indicate gas vesicle collapse and settling of Microcystis aeruginosa (Jančula et al. 2014, Thomas et al. 2019) sometimes accompanied by cell lysis (Thomas et al. 2019). Similarly, microcystin degradation is documented in some treatments (Medina, Griggs, and Thomas 2016, Thomas et al. 2019) but not others (Li, Song, and Yu 2014).
EFFECTIVENESS
- Water body type: Pond
- Surface area: Small
- Depth: Shallow
- Any trophic state, but typically most effective in eutrophic systems
- Mixing regime: Meromictic, monomictic, or dimictic
- Any water body use
NATURE OF HCB
- Surface-dwelling, gas-vesicle-containing HCBs
- Toxic and nontoxic HCBs; effective for cyanotoxins
- Intervention strategy
Cavitation yields free hydroxyl radicals and reactive oxygen species, oxidizing agents also produced in several other cyanobacteria in-lake prevention and intervention strategies, including barley and rice straw, the emerging nanobubbling technique, nanoparticles (for example, titanium dioxide), flocculation with clay and surfactant, and ultrasound treatments. Hydrodynamic cavitation, to date, has only been used in near-surface waters (a few feet) but has been suggested as a feasible approach for blooms that accumulate near the shore or in small lake embayments (Medina, Griggs, and Thomas 2016). Multiple cavitation cycles may be needed for maximum bloom loss: Jančula et al. (2014) reported that one cavitation cycle removed 66% of a natural surface Microcystis sp. bloom, followed by 73%, 83%, 94%, 97%, and 99% after two, four, six, 12, and 18 cycles, respectively. Li et al. (2015) also reported that hydrodynamic cavitation is superior to cavitation action caused by audio waves (ultrasound) in that 88% of M. aeruginosa was removed after 10 minutes, while only 39% was lost with ultrasound treatment.
ADVANTAGES
- Eliminates surface blooms and toxins
- Reported water quality and ecological benefits
- Effective on gas-vesicle-containing cyanobacteria with low impact on other phytoplankton
LIMITATIONS
- High costs
- Needs infrastructure (electricity, piping, boat ramp, etc.)
- With suboptimal treatment, cells may remain intact, fuel bottom BOD, and not oxidize toxins
- Treats only surface blooms; useful in small ponds
- Repeated treatments may be required throughout the growing season
CASE STUDY EXAMPLES
California, United States: Most recent work involves transferring natural blooms to mesocosms and subjecting these contained HCBs to hydrodynamic cavitation. Medina, Griggs, and Thomas (2016) worked on aliquots from natural blooms and noted 32% reductions in cell numbers using only hydrodynamic cavitation; however, with the addition of superoxide, 81% of initial cell numbers were removed vs. only 23% with just cavitation. In two lakes samples, microcystin concentrations declined 68% and 87% with hydrodynamic cavitation treatments, with only slightly higher declines (77% and 92%) when superoxide additions followed cavitation.
Lake Neatahwanta, New York, United States: In another pilot study with field blooms moved to the laboratory, Shaw (2020) reported a 50% reduction in cyanobacteria chlorophyll 72 hours after hydrodynamic cavitation treatment; if also treated with peroxide, the reduction was approximately 80%. Field trials of this approach are now underway.
COST ANALYSIS
Few field prototypes exist currently, but access to a bloom may require a boat, power for pumping lake water through the apparatus, and special equipment for microbubble generation. If other additives are included (for example, hydrogen peroxide or a superoxide generator), these will be additional expenses.
Relative cost per growing season: Hydrodynamic cavitation
| ITEM | RELATIVE COST PER GROWING SEASON |
| Material | $$ |
| Personal Protective Equipment | $$ |
| Equipment | $$$ |
| Machinery | $$ |
| Labor | $$ |
| O&M Costs | $$$ |
| OVERALL | $$$ |
REGULATORY AND POLICY CONSIDERATIONS
Because hydrodynamic cavitation is a new strategy, state officials should be contacted about permitting and application. If other oxidizing compounds are included with cavitation, the use of and training for these additional compounds should be explored, including their potential effects on applicators and other lake biota.
REFERENCES
Jančula, Daniel, Přemysl Mikula, Blahoslav Maršálek, Pavel Rudolf, and František Pochylý. 2014. “Selective method for cyanobacterial bloom removal: hydraulic jet cavitation experience.” Aquaculture International 22 (2):509-521. doi: 10.1007/s10499-013-9660-7.
Li, Pan, Yuan Song, and Shuili Yu. 2014. “Removal of Microcystis aeruginosa using hydrodynamic cavitation: Performance and mechanisms.” Water Research 62:241-248. doi: 10.1016/j.watres.2014.05.052.
Li, Pan, Yuan Song, Shuili Yu, and Hee-Deung Park. 2015. “The effect of hydrodynamic cavitation on Microcystis aeruginosa: Physical and chemical factors.” Chemosphere 136:245-251. doi: 10.1016/j.chemosphere.2015.05.017.
Medina, V. F., C. S. Griggs, and C. Thomas. 2016. “Evaluation of the destruction of the harmful cyanobacteria, Microcystis aeruginosa, with a cavitation and superoxide generating water treatment reactor.” Bull Environ Contam Toxicol 96 (6):791-6. doi: 10.1007/s00128-016-1742-6.
Shaw, S. 2020. Hydrodynamic cavitation with hydrogen peroxide. August 12, 2020. . In NYS Department of the Environment webinar. https://meetny.webex.com/recordingservice/sites/meetny/recording/play/f93ad725989b44849c8b8b0627494bbb.
Thomas, Catherine, Afrachanna Butler, C. S. Griggs, Victor Medina, and Allan Katzenmeyer. 2019. “Physicochemical Treatment of Cyanobacteria and Microcystin by Hydrodynamic Cavitation and Advanced Oxidation. ERDC/EL TR-19-2.” https://apps.dtic.mil/sti/pdfs/AD1068056.pdf.
Zhang, Guangming, Panyue Zhang, Hong Liu, and Bo Wang. 2006. “Ultrasonic damages on cyanobacterial photosynthesis.” Ultrasonics Sonochemistry 13 (6):501-505. doi: 10.1016/j.ultsonch.2005.11.001.
HYPOLIMNETIC OXYGENATION AND AERATION
In-lake Prevention Strategy
Substantial Supporting Data
Hypolimnetic oxygenation and aeration have been successfully used in lakes and reservoirs as physical controls to maintain oxygen levels in bottom waters while preserving thermal stratification and avoiding warming the hypolimnion Beutel and Horne (1999), (Bormans, Marsálek, and Jancula 2015, Visser et al. 2016). In the case of controlling HCBs, a hypolimnetic aeration or oxygenation system is designed to reduce concentrations of limiting nutrients, such as phosphorus, in the hypolimnion, with minimum mixing across the metalimnion to avoid the sudden introduction of nutrient-rich bottom waters into the epilimnion (Bormans, Marsálek, and Jancula 2015). Wagner (2015) presents a summary of oxygenation efficacies for reducing cyanobacteria across a suite of case studies.
Hypolimnetic oxygenation uses pure oxygen, whereas hypolimnetic aeration uses air to maintain oxygen levels and prevent the long-term storage of nutrients, encouraging natural cycling through the system rather than sudden entrainment into the epilimnion (Beutel and Horne 1999, Bormans, Marsálek, and Jancula 2015, Sahoo et al. 2015). Several types of hypolimnetic oxygenation or aeration systems slowly release oxygen or air using pumps, pipes, diffusers, or submerged chambers (Cooke et al. 2005). Systems are grouped into three categories: (1) mechanical agitation, (2) injection of pure oxygen, and (3) injection of air through a full lift design, partial lift design, or downflow injection design (Cooke et al. 2005). The use of mixers, aerators, and diffusers to oxygenate a hypolimnion or induce artificial mixing is fundamentally different from the strategies that employ nanobubbles and ozonation. Nanobubble and ozonation strategies induce synthetic biochemical reactions rather than reinforce inherent biological or physical processes.
EFFECTIVENESS
- Water body type: Lake/reservoir
- Any surface area
- Depth: Deep; requires large hypolimnion; avoid in shallow, unstratified systems
- Any trophic state, but typically most effective in eutrophic systems
- Mixing regime: Meromictic, monomictic, or mimictic
- Any water body use
- Watershed loading levels will impact effectiveness
EFFECTIVENESS
- Repeating HCBs
- Toxic and nontoxic HCBs; effective for cyanotoxins
- Targets all algal species
- Prevention strategy
These physical controls are most effective in systems that have or are expected to experience extensive, sustained nutrient and sediment loading and require remediation beyond periodic intervention strategies to protect the water quality and ecosystem (Bormans, Marsálek, and Jancula 2015). Often, hypolimnetic oxygenation is used in conjunction with watershed controls and algaecide treatments (Bormans, Marsálek, and Jancula 2015, Moore and Christensen 2009, Visser et al. 2016). Physical oxygenation or aeration methods may not operate satisfactorily if the water body is too shallow—even if stratification exists—as the density gradient may not be sufficient to resist thermocline attenuation while the hypolimnion is mixing (Bormans, Marsálek, and Jancula 2015). Physical oxygenation or aeration methods can cause a change in composition from cyanobacterial dominance to green algae and diatoms if the water body is deep enough to limit light availability and the oxygenation or aeration devices are well distributed horizontally over the lake (Bormans, Marsálek, and Jancula 2015, Visser et al. 2016).
ADVANTAGES
- No waste or by-products produced
- Readily available equipment
- Successful full-scale implementation
- Reported water quality and ecological benefits
- Indiscriminate of algae species
- Minimal aesthetic impact
LIMITATIONS
- High installation costs
- High operational costs associated with year-round use
- Needs infrastructure (electricity, piping, boat ramp, etc.)
- Limited scalability
- Potential water chemistry restrictions
- Potential sediment chemistry restrictions
- Potential unintentional biological impacts
Many examples of hypolimnetic aeration applications in lakes and reservoirs worldwide have been reported in the literature; extensive reviews include Beutel and Horne (1999), Cooke et al. (2005), and Singleton and Little (2007). Successful deployment of hypolimnetic oxygenation can delay stratification onset, establish a diatom population, allow this diatom population to persist longer, and remove limiting nutrients from the water column so that less nutrients are available in the epilimnion for cyanobacterial growth (Bormans, Marsálek, and Jancula 2015). Unsuccessful treatments that fail to mitigate HCBs are reported to have come from (1) inadequately sized aerators that do not account for increased BOD or increase diffusion of the limiting nutrient into the epilimnion, resulting in enhanced cyanobacterial growth; (2) low availabilities of trace metals required for limiting nutrient fixation; (3) lack of external load control; and (4) lack of sufficient operation time (Bormans, Marsálek, and Jancula 2015, National Research Council 2000). If these limitations are overcome, hypolimnetic aeration may reduce hypolimnetic nutrient accumulation and internal cycling and, ultimately, reduce the development of HCBs.
Adverse biological effects resulting from aeration have also been reported. Supersaturation of hypolimnetic water with N2 might lead to a gas bubble disease in fish in some cases (Kortmann, Knoecklein, and Bonnell 1994).
However, some biological and ecological benefits may also result from aeration. Aeration allows for deeper zooplankton distribution and refuge from predators in the dark bottom waters during the day (McComas 2003). In addition, the expanded aerobic environment may enhance growth and expansion of cold-water fish habitat and population due to increased oxygen concentrations, increased visibility, and greater zooplankton density (Rieberger and BC Environment 1992).
The following criteria are recommended by Bormans, Marsálek, and Jancula (2015), in agreement with those proposed by Schauser, Lewandowski, and Hupfer (2003) and Hickey and Gibbs (2009). You should consider these criteria before choosing a physical oxygenation or aeration mitigation strategy:
1. Define the critical limiting nutrient level needed to achieve the predicted outcome.
2. Assess the dynamics and relative role of internal nutrient loading compared to external loading.
3. Assess the sediment characteristics to determine whether internal loading can be controlled.
4. Quantify the link between internal load and cyanobacterial biomass.
5. Scale the treatment as a function of the internal load and the size of the lake.
6. Evaluate the potential to cause adverse effects to aquatic biota.
7. Set a long-term monitoring program before, during, and after the treatment.
COST ANALYSIS
The costs of installing and maintaining a hypolimnetic oxygenation or aeration system are relatively high, mostly due to operating costs associated with the generally continuous operation for successful applications. Costs are also dependent on the type of equipment and local power rates (Bormans, Marsálek, and Jancula 2015). Increased availability and performance of photovoltaic technologies may help mediate power costs. Aerators are usually installed in spring and run during the whole summer (growing) season until autumn (Bormans, Marsálek, and Jancula 2015). The costs associated with this method are not often reported in the literature. Costs of oxygen injection estimated by Hickey and Gibbs (2009) were around $2,500/ha/year, while Cooke et al. (2005) reported overall costs for an average of 15 lakes in the United States of $3,000/ha/year. A hypolimnetic aeration system installed in the late 1990s in Amisk, Canada, reported capital costs of $30,000 and operating costs of about $49,000/year (Prepas and Burke 2011). Procedures for sizing hypolimnetic aerators, and thus determining lake-specific cost estimates, are described in detail by Ashley (1985), Little (1995), Lorenzen and Fast (1977). Other estimates can be found in Appendix C.2.
CASE STUDY EXAMPLES
Newman Lake, Washington, United States: In the late 1960s and early 1970s, summer and fall blooms of cyanobacteria began to occur in Newman Lake. Through the next decade, these blooms intensified and became an annual occurrence. A restoration feasibility assessment of the lake and watershed indicated that a major portion of phosphorus loading (∼83%) was attributable to internal recycling associated with summer hypolimnetic oxygen depletion. In 1972, a Speece cone for hypolimnetic oxygenation was installed to supplement watershed controls and alum treatments. More details are provided in (Moore and Christensen 2009).
Average summer volume-weighted total phosphorus declined from pre-restoration levels exceeding 50 μg-P/L to an average of 21 μg-P/L over 7 years. Most notably, peak annual biovolumes of cyanobacteria and their representation within the phytoplankton community decreased substantially, with increased prevalence of diatoms and green and golden-brown algae.
Overall, the response to nutrient reduction at Newman Lake is consistent with worldwide observations that emphasize the need for long-term perspectives and commitment in lake restoration and management. Continuation of internal load controls and increased emphasis on external nutrient abatement have been implemented to supplement positive water quality trends, despite future development increases and land use changes.
Relative cost per growing season: Hypolimnetic oxygenation and aeration
| ITEM | RELATIVE COST PER GROWING SEASON |
| Material | $$ |
| Personal Protective Equipment | $ |
| Equipment | $$$ |
| Machinery | $$$ |
| Labor | $$ |
| O&M Costs | $$$ |
| OVERALL | $$$ |
REGULATORY AND POLICY CONSIDERATIONS
Before implementing a management action, you should establish a cause–effect linkage between the problem and the proposed management approach (Hickey and Gibbs 2009, USEPA 2000). Because multiple stressors and environmental factors frequently combine to cause the effects observed in aquatic ecosystems, an integrated approach with multiple management measures is often required to holistically address ecological issues in lakes. The decision to introduce a hypolimnetic oxygenation system should be based on a thorough understanding of the factors contributing to recurrent blooms and preliminary research to establish that an artificial oxygenation approach is a feasible option for reducing the frequency and severity of HCBs.
Following Hickey and Gibbs (2009), the preliminary work would involve:
- Characterizing the main drivers likely to be responsible for the HCBs occurring in the lake. Specifically, information would be needed on:
- The physical characteristics of the lake:
- volume
- depth
- clarity
- stratification
- deoxygenation (including duration of anaerobic conditions)
- annual variation in the concentrations of major nutrients
- input and output budgets for the major nutrients
- annual changes in algal biomass and species
- information on geothermal inflows
- The physical characteristics of the lake:
- Determining the stratification classification and assessing whether the lake forms a stable stratification (USDA 1999).
- Determining that sediments will release nutrients under realistic conditions, particularly anaerobic conditions (sediment core measurements or hypolimnetic nutrient measurements).
- Considering other potential treatment options to address internal nutrient loading, including:
- hydraulic flushing
- sediment dredging
- other source control measures, such as phosphorus-binding agents
This assessment must also include social and cultural values that need to be considered on a case-by-case basis with public and multi-agency consultation, which may uncover concerns with a specific product or approach. The selection and decision-making process may need to be modified accordingly. Any supplementary watershed controls or algaecide treatments must comply with policies and regulations as enacted by the appropriate oversight agency or authority. For some lakes, additional approval may be required from the U.S. Fish and Wildlife Service and the National Oceanic and Atmospheric Administration’s National Marine Fisheries Service under the ESA if endangered, threatened, or otherwise special status species are present, or if the lakes are in conservation land (USFWS 2020). Special consideration for protection of native or indigenous species may be made.
REFERENCES
Ashley, Kenneth Ian. 1985. “Hypolimnetic aeration: practical design and application.” Water Research 19 (6):735-740. doi: 10.1016/0043-1354(85)90120-4.
Beutel, Marc W., and Alex J. Horne. 1999. “A review of the effects of hypolimnetic oxygenation on lake and reservoir water quality.” Lake and Reservoir Management 15 (4):285-297. doi: 10.1080/07438149909354124.
Bormans, Myriam, Blahoslav Marsálek, and Daniel Jancula. 2015. “Controlling internal phosphorus loading in lakes by physical methods to reduce cyanobacterial blooms: a review.” Aquatic Ecology 50. doi: 10.1007/s10452-015-9564-x.
Cooke, G., E. Welch, S. Peterson, and S. Nichols. 2005. Restoration and Management of Lakes and Reservoirs. Boca Raton: CRC Press.
Gantzer, P. Al, S. McCord, and K. S. Johnson. 2016. “Oxygenation of a Sierra Reservoir.” California Lake Management Society Annual Conference, Davis, CA, October 13-14, 2016.
Hickey, Christopher W., and Max M. Gibbs. 2009. “Lake sediment phosphorus release management—Decision support and risk assessment framework.” New Zealand Journal of Marine and Freshwater Research 43 (3):819-856. doi: 10.1080/00288330909510043.
Kortmann, Robert W., George W. Knoecklein, and Charles H. Bonnell. 1994. “Aeration of stratified lakes: theory and practice.” Lake and Reservoir Management 8 (2):99-120. doi: 10.1080/07438149409354463.
Little, John C. 1995. “Hypolimnetic aerators: predicting oxygen transfer and hydrodynamics.” Water Research 29 (11):2475-2482. doi: 10.1016/0043-1354(95)00077-X.
Lorenzen, M. , and A. Fast. 1977. ” A guide to aeration/circulation techniques for lake management. EPA/600/3-77/004.” Washington, D. C. . https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=ORD&direntryid=44444.
McComas, Steve. 2003. Lake and pond management guidebook. Boca Raton, FL: Lewis Publishers.
Moore, Barry C., and David Christensen. 2009. “Newman Lake restoration: A case study. Part I. Chemical and biological responses to phosphorus control.” Lake and Reservoir Management 25 (4):337-350. doi: 10.1080/07438140903172907.
National Research Council. 2000. Clean Coastal Waters: Understanding and Reducing the Effects of Nutrient Pollution. Washington, DC: The National Academies Press.
Prepas, E., and J. Burke. 2011. “Effects of hypolimnetic oxygenation on water quality in Amisk Lake, Alberta, a deep, eutrophic lake with high internal phosphorus loading rates.” Canadian Journal of Fisheries and Aquatic Sciences 54:2111-2120. doi: 10.1139/f97-125.
Rieberger, K., and BC Environment. 1992. “Metal Concentrations in Fish Tissue from Uncontaminated B.C. Lakes.” Victoria: Water Quality Section. https://books.google.com/books?id=OLG3AAAACAAJ.
Sahoo, G., Alexander Forrest, S. Schladow, J. Reuter, Robert Coats, and Michael Dettinger. 2015. “Climate change impacts on lake thermal dynamics and ecosystem vulnerabilities.” Limnology and Oceanography 61:n/a-n/a. doi: 10.1002/lno.10228.
Schauser, I., J. Lewandowski, and M. Hupfer. 2003. “Decision support for the selection of an appropriate in-lake measure to influence the phosphorus retention in sediments.” Water Res 37 (4):801-12. doi: 10.1016/s0043-1354(02)00439-6.
Singleton, Vickie, and John Little. 2007. “Designing Hypolimnetic Aeration and Oxygenation Systems − A Review.” Environmental Science & Technology 40:7512-20. doi: 10.1021/es060069s.
USDA. 1999. “A Procedure to Estimate the Response of Aquatic Systems to Changes in Phosphorus and Nitrogen Inputs.” Portland, OR: U. S. Department of Agriculture, Natural Resources Conservation Service, National Water and Climate Center. https://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/stelprdb1044774.pdf.
USEPA. 2000. “Stressor Identification Guidance Document EPA 822-B-00-025.” U. S. Environmental Protection Agency, Office of Water. https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=20685.
USFWS. 2020. “Endangered Species Laws and Policies.” U. S. Fish and Wildlife Service, Ecological Services. https://www.fws.gov/sites/default/files/documents/endangered-species-act-accessible.pdf.
Visser, Petra M, Bas W Ibelings, Myriam Bormans, and Jef Huisman. 2016. “Artificial mixing to control cyanobacterial blooms: a review.” Aquatic Ecology 50 (3):423-441.
Wagner, Kenneth J. . 2015. “Oxygenation and Circulation to Aid Water Supply Reservoir Management.” Water Research Foundation. https://books.google.com/books/about/Oxygenation_and_Circulation_to_Aid_Water.html?id=hbwkrgEACAAJ.
HYPOLIMENTIC WITHDRAWAL AND DRAWDOWN
In-lake Prevention Strategy
Substantial Supporting Data
It is well established that vertical water column stability and long water residence times favor cyanobacteria over eukaryotic phytoplankton (Ibelings et al. 2016, Paerl et al. 2016, Mitrovic et al. 2003). Thus, the disruption of these conditions can, under certain circumstances, reduce nuisance HCBs (Havens et al. 2019, Lehman 2014, McDonald and Lehman 2013). Management strategies that decrease water levels or selectively release nutrient-rich bottom waters can be effective management tools that affect nutrient delivery to HCBs (Paerl et al. 2016). The geographic setting of the water body and lake depth will dictate which type of in-lake management strategy is feasible based on water availability or lack thereof. For example, arid western regions of the United States may have more restrictions than eastern to midwestern regions.
Hypolimnetic withdrawal is the removal of nutrient-rich bottom waters in stratified ponds and lakes to eliminate nutrient supplies that support the growth of cyanobacteria in the epilimnion (surface layer) of the water body. Bormans, Marsálek, and Jancula (2015) have reviewed this strategy and its use in multiple lakes and report variable results. It has been successful in eliminating blooms of Aphanizomenon in Ford Lake, Michigan, while not having any effect on Microcystis or microcystin toxicity (Lehman, McDonald, and Lehman 2009). Further, destabilization led to diatom prevalence (McDonald and Lehman 2013). In Lake Mauensee in Switzerland, Gächter (1976) reported the disappearance of Planktothrix rubescens following withdrawal. This cyanobacterium was also reduced in a Slovenian lake following withdrawal and reduction in external loads (Vrhovšek et al. 1985).
Water level fluctuations (drawdown) may be defined as the lowering of the water level to expose littoral zone habitat and sediments, with the goal to switch the water body from a turbid, algae-dominated system to a clear-water, plant-dominated system (Scheffer et al. 1993). However, the timing of the water level drawdown is critical, because summer-time drawdowns can increase cyanobacteria production given the increased water retention time, increased water temperature, and nutrients (Bakker and Hilt 2016). In shallow lakes, lower water elevations at key times of the year may promote the growth of submerged and emergent macrophytes due to increased light availability and reduce the potential for cyanobacteria development (Coops and Hosper 2002, Scheffer and van Nes 2007). Mechanisms that indirectly affect cyanobacteria development during drawdown include exposure of over-wintering cyanobacteria populations on surficial sediments to winter freezes, disruption and loss of colonizable habitat for benthic cyanobacteria (Turner et al. 2005), uptake of nutrients by macrophytes, excretion of allelopathic substances by macrophytes that may inhibit cyanobacteria growth (Hilt and Gross 2008), or development of macrophyte beds that support invertebrate and fish assemblages (Bakker and Hilt 2016). In contrast, water level drawdown is often used in deeper lakes to reduce aquatic nuisance plants and fish. Due to the timing of drawdown (for example, winter), the strategy generally limits the effectiveness of managing cyanobacteria blooms.
EFFECTIVENESS
- Water body type: Lake/reservoir
- Any surface area
- Depth: Deep; requires large hypolimnion; avoid in shallow, unstratified systems
- Any trophic state, but typically most effective in eutrophic systems
- Mixing regime: Meromictic, monomictic, or dimictic
- Any water body use
- Watershed loading levels will impact effectiveness
NATURE OF HCB
- Repeating HCBs
- Toxic and nontoxic HCBs
- Hypolimnetic withdrawal targets several species
- Drawdown is more effective on benthic cyanobacteria (for example, Planktothrix)
- Prevention strategy
As a control strategy, hypolimnetic withdrawal from stratified systems is most effective in systems where internal nutrient loads are the primary cause of the HCB and external nutrient loads are declining or low. Withdrawal can result in destratification and increases in NO3 deeper in the water column. Further, there may be total phosphorous concentration thresholds for some species. Bormans, Marsálek, and Jancula (2015) reported that cyanobacteria declined when epilimnion total phosphorus levels were less than 25 µg/L. This might suggest that hypolimnion total phosphorus levels >25 µg/L could be an indicator for selecting use of withdrawal as a strategy to consider in HCB control. In addition, Lehman, McDonald, and Lehman (2009) noted that Aphanizomenon was found when the total nitrogen/total phosphorus ratio approximated 48, while Microcystis was common at ratios approximating 70. Other metrics for assessing whether cyanobacteria (or non-cyanobacteria) could follow hypolimnetic withdrawal; however, successful reductions in cyanobacteria may not always occur (see Table 1 in Bormans, Marsálek, and Jancula 2015, Dunalska et al. 2014).
Withdrawal can be accomplished through pumping sub-thermocline water from depth into downstream areas. A special withdrawal tube—an Olszewski pipe, with openings set at depths below the thermocline—has been used in the past. In lakes or reservoirs with dam outlets at depth, if those outlets are deeper than the thermocline, then opening the outlets following stratification and nutrient accumulation at depth could remove the regenerated nitrogen and phosphorus, thereby limiting access by cyanobacteria populations in the epilimnion.
At Milford Reservoir in Kansas, which has a surface area of over 15,000 acres, the management plan implemented since 2017 incorporates a spring drawdown that exposes a broad shallow area in the upper portion of the water body; this is specifically designed to reduce habitat where cyanobacterial blooms develop (USACE 2019).
ADVANTAGES
- No waste or by-products produced
- Readily available equipment
- Reported water quality and ecological benefits
- Minimal aesthetic impact
- Run-of-the river reservoirs may lend better characteristics for the routing of water with bottom withdrawal to supplement convective mixing and to reduce HCBs
- Successive winter drawdowns may improve trophic conditions the following summer and reduce the potential for HCBs
LIMITATIONS
- High installation costs
- High operational costs when pumping from depth is required
- If no deep water outlets are in the water body, there are infrastructure needs (electricity, piping)
- Potential downstream discharge issues, including water quality, smell, fueling downstream blooms, and delivery of HCBs and cyanotoxins during flushing events
- Not practical or effective on larger reservoirs
- Drawdown may decrease shoreline stability and increase erosion and sediment deposition
- Effectiveness of reservoir drawdown may depend on sediment characteristics and the potential for nutrient release from sediment and macrophytes upon rewetting
Regional rainfall patterns may impact capability, influence water residence time, and change cyanobacteria dominance and persistence (Jagtman, Van der Molen, and Vermij 1992, Larsen et al. 2020). Other environmental factors—such as thermal stratification, water temperature, and potential fisheries—should be considered before implementing this strategy (Fulton III and Hendrickson 2011, Nelson et al. 2018). Often, numerical modeling can help evaluate these environmental factors and determine whether hypolimnetic withdrawal or drawdown will be beneficial for the reservoir. The cost of raw water and limited supplies in many regions of the United States may also be a deciding factor. In these cases, the intangible cost (economics) of closing a water body due to HCBs should also be considered.
COST ANALYSIS
Relative cost per growing season: Hypolimnetic withdrawal
| ITEM | RELATIVE COST PER GROWING SEASON |
| Material | $$ |
| Personal Protective Equipment | $ |
| Equipment | $$ |
| Machinery | $$ |
| Labor | $ |
| O&M Costs | $$ |
| OVERALL | $$ |
CASE STUDY EXAMPLES
Ford Lake, Michigan, United States: In 2011, the selective withdrawal of hypolimnetic water at a rate of approximately 80 MGD reduced potential power generation, resulting in a revenue loss for the township of approximately $355 per day. Because Ford Lake is a run-of-the river dam, a constant lake elevation is maintained with the need to discharge episodic rainfall events via the bottom withdrawal outlet. If hypolimnetic anoxia occurred prior to the selective withdrawal, then there would have been a greater risk downstream of poorer water quality or potentially fish kills (Lehman 2014).
Despite the limitations on selective withdrawal, elected officials decided to continue the practice of selective withdrawal, which resulted in a revenue loss of approximately $20,000 per year. The public’s willingness to accept financial tradeoffs for benefits in water quality led to summer withdrawals from 2009 to 2011 that reduced cyanobacteria blooms during this period. The selective withdrawal of hypolimnetic water enhanced the vertical mixing of the water column, limiting the cyanobacteria’s preferred habitat in the epilimnion.
Lake Mauensee, Switzerland, and Lake Bled, Slovenia: Hypolimnetic withdrawal resulted in disappearance and declines, respectively, of Planktothrix rubescens (Gächter 1976, Vrhovšek et al. 1985).
Kortowskie Lake, Poland: In contrast to the declines in cyanobacteria noted above, hypolimnetic withdrawal from Kortowskie Lake resulted in cyanobacteria increases in the metalimnion as well as overall lake productivity (Dunalska et al. 2014).
Financial costs depend on site-specific geographical settings and water availability. For example, if hydroelectric facilities are associated with run-of-the river facilities, the financial tradeoffs of water, electric power, and public perception must be thoroughly vetted before hypolimnetic withdrawal or drawdown management strategies are implemented. In the arid West, water availability and the cost of water severely limit the feasibility of hydraulic or flushing strategies, although water level drawdown may be more practical in this region.
REGULATORY AND POLICY CONSIDERATIONS
Nearly all in-lake prevention or intervention techniques, including hypolimnetic withdrawal and water level drawdown, will require some form of permitting or approval at the federal, state, or local level (Holdren, Jones, and Taggart 2001). Because these management strategies have the potential to flush sediment, nutrients, cyanobacteria (cyanotoxins), and other metalloid or hydrocarbon compounds to downstream regulated water bodies (as well as affect streamflow and water availability downstream), the state water quality regulatory office is the most appropriate agency to contact early in the planning phase.
Regulatory planning for hypolimnetic withdrawal or drawdown techniques may include but is not limited to Clean Water Act Sections 401 or 404 permitting, NPDES permitting, drawdown permitting, and Water Rights Administration permitting. Depending on the scale of the project and the extent of stakeholders, permitting could take months to years, so planning is critical. Depending on the size of the water body, its physical characteristics, and its environmental setting, implementing these techniques as short-term intervention approaches may require extensive planning. Local and state officials should be contacted regarding permitting and use, particularly for potential impacts downstream from nutrient-rich, potentially sulfidic bottom waters.
REFERENCES
Bakker, Elisabeth S., and Sabine Hilt. 2016. “Impact of water-level fluctuations on cyanobacterial blooms: options for management.” Aquatic Ecology 50 (3):485-498. doi: 10.1007/s10452-015-9556-x.
Bormans, Myriam, Blahoslav Marsálek, and Daniel Jancula. 2015. “Controlling internal phosphorus loading in lakes by physical methods to reduce cyanobacterial blooms: a review.” Aquatic Ecology 50. doi: 10.1007/s10452-015-9564-x.
Coops, Hugo, and S. Harry Hosper. 2002. “Water-level management as a tool for the restoration of shallow lakes in the Netherlands.” Lake and Reservoir Management 18 (4):293-298. doi: 10.1080/07438140209353935.
Dunalska, Julita A., Peter A. Staehr, Bożena Jaworska, Dorota Górniak, and Piotr Gomułka. 2014. “Ecosystem metabolism in a lake restored by hypolimnetic withdrawal.” Ecological Engineering 73:616-623. doi: 10.1016/j.ecoleng.2014.09.048.
Fulton III, R. S., and J. C. Hendrickson. 2011. Relationships of Water Flow with Plankton and Water Quality In St. Johns River Water Management District. Palatka, FL.
Gächter, V.R. . 1976. “Die Tiefenwasserableitung, ein Weg zur Sanierung von Seen.” Swiss J. Hydrol. 38:1-29.
Havens, Karl E., Gaohua Ji, John R. Beaver, Rolland S. Fulton, and Catherine E. Teacher. 2019. “Dynamics of cyanobacteria blooms are linked to the hydrology of shallow Florida lakes and provide insight into possible impacts of climate change.” Hydrobiologia 829 (1):43-59. doi: 10.1007/s10750-017-3425-7.
Hilt, Sabine, and Elisabeth Gross. 2008. “Can allelopathically active submerged macrophytes stabilise clear-water states in shallow lakes?” Basic and Applied Ecology 9:422-432. doi: 10.1016/j.baae.2007.04.003.
Holdren, C. , W. Jones, and J. Taggart. 2001. “Managing Lakes and Reservoirs.” North American Lake Management Society; Terrene Insitute. https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=20004KKC.txt.
Ibelings, Bastiaan W., Myriam Bormans, Jutta Fastner, and Petra M. Visser. 2016. “CYANOCOST special issue on cyanobacterial blooms: synopsis—a critical review of the management options for their prevention, control and mitigation.” Aquatic Ecology 50 (3):595-605. doi: 10.1007/s10452-016-9596-x.
Jagtman, E., D.T. Van der Molen, and S Vermij. 1992. ” The influence of flushing on nutrient dynamics, composition and densities of algae and transparency in Veluwemeer, The Netherlands.” In Restoration and Recovery of Shallow Eutrophic Lake Ecosystems in The Netherlands. Developments in Hydrobiology, edited by L. Van Liere and R.D. Gulati. Dordrecht: Springer.
Larsen, Megan L., Helen M. Baulch, Sherry L. Schiff, Dana F. Simon, Sébastien Sauvé, and Jason J. Venkiteswaran. 2020. “Extreme rainfall drives early onset cyanobacterial bloom.” bioRxiv:570275. doi: 10.1101/570275.
Lehman, E. M., K. E. McDonald, and J. T. Lehman. 2009. “Whole lake selective withdrawal experiment to control harmful cyanobacteria in an urban impoundment.” Water Res 43 (5):1187-98. doi: doi: 10.1080/07438140903117217.
Lehman, John T. 2014. “Understanding the role of induced mixing for management of nuisance algal blooms in an urbanized reservoir.” Lake and Reservoir Management 30 (1):63-71. doi: 10.1080/10402381.2013.872739.
McDonald, Kahli E., and John T. Lehman. 2013. “Dynamics of Aphanizomenon and Microcystis (cyanobacteria) during experimental manipulation of an urban impoundment.” Lake and Reservoir Management 29 (2):103-115. doi: 10.1080/10402381.2013.800172.
Mitrovic, S. M., R. L. Oliver, C. Rees, L. C. Bowling, and R. T. Buckney. 2003. “Critical flow velocities for the growth and dominance of Anabaena circinalis in some turbid freshwater rivers.” Freshwater Biology 48 (1):164-174. doi: 10.1046/j.1365-2427.2003.00957.x.
Nelson, Natalie G., Rafael Muñoz-Carpena, Edward J. Phlips, David Kaplan, Peter Sucsy, and John Hendrickson. 2018. “Revealing biotic and abiotic controls of harmful algal blooms in a shallow subtropical lake through statistical machine learning.” Environmental Science & Technology 52 (6):3527-3535. doi: 10.1021/acs.est.7b05884.
Paerl, H. W., W. S. Gardner, K. E. Havens, A. R. Joyner, M. J. McCarthy, S. E. Newell, B. Qin, and J. T. Scott. 2016. “Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients.” Harmful Algae 54:213-222. doi: 10.1016/j.hal.2015.09.009.
Scheffer, M., S. H. Hosper, M. L. Meijer, B. Moss, and E. Jeppesen. 1993. “Alternative equilibria in shallow lakes.” Trends in Ecology & Evolution 8 (8):275-279. doi: 10.1016/0169-5347(93)90254-M.
Scheffer, Marten, and Egbert H. van Nes. 2007. “Shallow lakes theory revisited: various alternative regimes driven by climate, nutrients, depth and lake size.” Hydrobiologia 584 (1):455-466. doi: 10.1007/s10750-007-0616-7.
Turner, Michael A, David B Huebert, David L Findlay, Leonard L Hendzel, Wolfgang A Jansen, RA Bodaly, Llwellyn M Armstrong, and Susan EM Kasian. 2005. “Divergent impacts of experimental lake-level drawdown on planktonic and benthic plant communities in a boreal forest lake.” Canadian Journal of Fisheries and Aquatic Sciences 62 (5):991-1003. doi: 10.1139/f05-003.
USACE. 2019. “Lake level management plans water year 2020.” U. S. Army Corps of Engineers, Kansas City District. https://kwo.ks.gov/docs/default-source/reservoirs/rpt_llmp_wy2020_12312019_tj.pdf?sfvrsn=d0518214_0.
Vrhovšek, D., G. Kosi, M. Kralj, M. Bricelj, and M. Zupan. 1985. “The effect of lake restoration measures on the physical, chemical and phytoplankton variables of Lake Bled.” Hydrobiologia 127 (3):219-228. doi: 10.1007/BF00024227.
MICROBIAL BIOMANIPULATION
In-lake Intervention Strategy
Emerging Supporting Field Data
Viruses, fungi, protozoa, and indigenous bacteria have been suggested as agents that can remove cyanobacterial cells and toxins from the water column via a broad range of mechanisms (Sigee et al. 1999, Yoshida et al. 2008). Some bacteria may settle cyanobacteria out of the water column by aggregation or bioflocculation. Other bacteria and viruses may lyse (break open) cyanobacteria cells; still other bacteria may degrade microcystins and perhaps other cyanotoxins. A relatively new hybrid application involves using microporous bubbling aeration techniques to destratify the lake and reoxygenate deep bottom waters, followed by seeding the bottom sediments with bacteria or enzyme mixtures to oxidize settled cyanobacteria and reduce the availability of recycled nutrients that would support cyanobacteria regrowth. The hybrid treatment appears to be most effective when destratification and bottom organic matter oxidation is followed by the addition of micronutrients that favor the growth of non-cyanobacteria. There is concern, however, that the introduction of non-native or engineered bacteria may have unforeseen and irreversible consequences, such as altering bacterial communities and processes that drive ecosystem dynamics.
Multiple bacteria and several viruses, fungi, and protozoa have been isolated that, in the laboratory, lyse bloom-forming cyanobacteria (Jiang et al. 2019) and degrade toxins (Li, Li, and Li 2017) for microcystins. These potential biological control agents include members of the Bacteroides-Cytophaga-Flavobacterium complex, specifically Bacillus spp., Flexibacter spp., Cytophaga, and Myxobacteria (Gumbo, Ross, and Cloete 2008). For these bacteria to be used for biocontrol, they must have densities approximating 106/mL and complement high cyanobacteria abundances, ensuring close contact between the two populations. In the laboratory, Nakamura et al. (2003) inoculated a “floating carrier” of biodegradable, starch-based plastic with Bacillus cereus N-14. The addition yielded a 99% decline in planktonic cyanobacteria in 4 days; without the carrier, the decline was only 7.5%.
Attaining high population densities of desirable bacteria in small volumes should be relatively inexpensive, since the methods to culture bacteria are well known and can be readily applied. However, scaling to the volumes of bacteria needed for whole-lake application would be expensive. Wang et al. (2020) described the use of bacteria as a control because of their “potential effectiveness, species specificity, and eco-friendly characteristics.” While using bacteria to control blooms may eventually be a cost-effective, safe treatment, timing for posting the treatment for general use in a lake for recreation or drinking water is unknown. Since exocellular polysaccharides are also produced by bacteria, a non-contact period for recreational waters might be considered to avoid potential allergic reactions to these by-products. In addition, cyanotoxin analyses should occur, as toxins can be released when cyanobacteria cells die, are lysed, or settle out of the water column and break down in the sediments. This might be mitigated through the addition of a second microcystin-degrading bacterium assemblage or other treatment agents (for example, oxidation agents such as peroxide or ozone).
EFFECTIVENESS
- Water body types: Pond, lake/reservoir
- Surface area: Small
- Depth: Deep
- Trophic status: Eutrophic
- Any mixing regime
- Alkaline systems
- Water body uses: Recreation, drinking water
- Confined to bloom area or isolated coves
NATURE OF HCB
- Surface bloom of cyanobacteria (Microcystis aeruginosa is a good candidate)
- Toxic and nontoxic HCBs
- Intervention strategy
The use of bacteria, viruses, fungi, or protozoa for cyanobacteria removal requires a surface cyanobacteria bloom, a high density of the effective biological agent, and interventions to ensure high bioagent–cyanobacteria contact (for example, bioflocculation or floatation carriers). A section of a lake can be isolated (for example, a cove on the windward side of the lake or vertical weir curtains dropped in a lake).
ADVANTAGES
- Unlikely carry-over after bloom dissipation, as the added bacteria or other microbial agent can then shift to a different energy source
- Low potential for adverse impacts if indigenous isolates are used
LIMITATIONS
- Very limited field use to date
- Needs a laboratory to culture the large volumes of effective isolates, a boat for delivery, and floating inoculated substrates
- Limited toxicity information for cultured isolates
- Cyanotoxin control may be limited; only microcystin degradation has been studied
- Surface water criteria concerns for toxin release as cells lyse
- Permitting requirements unknown
- Potential long-term, irreversible ecosystem impacts if non-indigenous isolates are used
COST ANALYSIS
Relative cost per growing season: Microbial biomanipulation
| ITEM | RELATIVE COST PER GROWING SEASON |
| Material | $$$ |
| Personal Protective Equipment | $$ |
| Equipment | $$$ |
| Machinery | $$ |
| Tools | $$ |
| Labor | $$$ |
| O&M Costs | $$ |
| OVERALL | $$$ |
No cost projections are readily available, but initial costs would be high for culturing equipment (large-volume vats, autoclaves, incubators, glassware, media, and expendables). There would be costs for preparing starch-based carriers and methods and space for inoculating these substrates. The use or reuse of vertical weir curtains to separate water bodies further increases costs. Staffing and time demands would be substantial.
REGULATORY AND POLICY CONSIDERATIONS
Permitting requirements are unknown, but adding live isolates (bacteria, viruses, fungi, or protozoa) to natural waters requires evaluation.
REFERENCES
Gumbo, Jabulani, Gina Ross, and Thomas Cloete. 2008. “Biological control of Microcystis dominated harmful algal blooms.” African Journal of Biotechnology 7.
Jiang, Xuewen, Chanhee Ha, Seungjun Lee, Jinha Kwon, Hanna Cho, Tyler Gorham, and Jiyoung Lee. 2019. “Characterization of cyanophages in lake erie: interaction mechanisms and structural damage of toxic cyanobacteria.” Toxins 11 (8):444. doi: 10.3390/toxins11080444.
Li, Jieming, Renhui Li, and Ji Li. 2017. “Current research scenario for microcystins biodegradation – A review on fundamental knowledge, application prospects and challenges.” Science of The Total Environment 595:615-632. doi: 10.1016/j.scitotenv.2017.03.285.
Nakamura, N., K. Nakano, N. Sugiura, and M. Matsumura. 2003. “A novel control process of cyanobacterial bloom using cyanobacteriolytic bacteria immobilized in floating biodegradable plastic carriers.” Environ Technol 24 (12):1569-76. doi: 10.1080/09593330309385703.
Sigee, D., R. Glenn, M. Andrews, Edward Bellinger, R. Butler, H. Epton, and R. Hendry. 1999. “Biological control of cyanobacteria: Principles and possibilities.” Hydrobiologia 395-396:161-172. doi: 10.1023/A:1017097502124.
Wang, Meng, Shibao Chen, Wenguang Zhou, Wenqiao Yuan, and Duo Wang. 2020. “Algal cell lysis by bacteria: A review and comparison to conventional methods.” Algal Research 46:101794. doi: 10.1016/j.algal.2020.101794.
Yoshida, Mitsuhiro, Takashi Yoshida, Aki Kashima, Yukari Takashima, Naohiko Hosoda, Keizo Nagasaki, and Shingo Hiroishi. 2008. “Ecological dynamics of the toxic bloom-forming cyanobacterium Microcystis aeruginosa and its cyanophages in freshwater.” Applied and Environmental Microbiology 74 (10):3269-3273. doi: 10.1128/aem.02240-07.
MONITORED NATURAL ATTENUATION
In-lake Intervention Strategy
Substantial Supporting Field Data
HCBs go through natural growth and die-off cycles, often driven by seasons (Yamamoto and Nakahara 2009). Consider monitored natural attenuation (MNA) for the water body if your community is interested in more passive and less costly HCB management strategies. MNA may be feasible for an HCB if exposure risks can be controlled. Even if a more active approach is preferred, in certain cases MNA may be the only practical option—for example, if the affected water body is too remote or too large to be cost-effectively treated through an imposed engineered solution. Similarly, if the HCB occurs late in the growing season or after the recreational season is over, there may not be support or funding to invest the resources needed for active bloom treatment and management. On the other hand, if the water body is used as a drinking water source, MNA may not be an option (see Section 5).

Figure C-7. Signage instructing citizens not to drink pond water.
Source: Eric Roberts, 2019. Used with permission.
MNA is fundamentally a risk management strategy. This means that stakeholders will need to be comfortable temporarily living with a controlled level of risk. It also means that the risks will need to be regularly reassessed as the character and toxicity of the bloom changes through its life cycle—and as uses of and exposures to the water body evolve seasonally. Cyanotoxins also have variable persistence in natural systems, from days for anatoxin-a to over 200 years for microcystin in lake sediments (Stevens and Krieger 1991, Zastepa et al. 2017). Depending on stakeholder use of the affected water body, varying degrees of control measures may be needed to mitigate potential exposure pathways. For example, if the bloom-affected water is within a sparsely populated residential community or remote, isolated areas, posting warning signs along the shore may be adequate. However, in more densely populated communities, signage will probably need to be accompanied by reoccurring advertising on webpages or in community publications, email distributions, homeowner association member portals, or newspapers. More concepts and approaches for keeping the public informed can be found in Section 5.
A successfully and safely implemented MNA approach will likely include several key elements:
- Defining the problem: To adequately address the problem, answer questions like:
- What is the dominant cyanobacterium or cyanobacteria? Can it/they be expected to attenuate as planned?
- Where is the bloom in the water column (surface scum, subsurface layer, or dispersed)?
- How does the dominant cyanobacterium or cyanobacteria respond to expected seasonal changes?
- Is it reasonable to expect that the bloom will attenuate?
- Are cyanotoxins present?
- In what part of the growing season is the bloom occurring?
- Identifying and controlling exposure risks: You must also find the answers to questions like:
- Is the lake a drinking water reservoir?
- Is the lake used for swimming?
- Is the lake used for fishing? If so, is it a catch and release lake?
- Is the lake in a remote location, or in a populated area with domesticated animals?
- Do livestock have access to the water?
- Is wildlife exposure to the bloom a concern?
- Can signage and other communication tools be expected to adequately inform the public?
- Is the bloom occurring during the recreational season?
- Are there other news or social media means of effectively communicating exposure risks to the community?
- Monitoring the bloom and protective controls: Regularly monitor and test the water to answer questions like:
- How are cyanobacteria counts changing?
- What changes are occurring in dominant cyanobacteria species?
- Are cyanotoxins being produced? If so, are they at levels of concern?
- Is the bloom causing any unforeseen problems?
- Are there indications the bloom will not attenuate when expected?
- Is signage being maintained?
- Are public notices or other communications continuing?
- Is the public adhering to advisories?
- Is public sentiment changing?
Bloom monitoring generally includes tracking cyanobacteria population densities, species prevalence, and the presence and concentrations of toxins. USEPA (2019) recommends a sequential approach to monitor blooms. Initially, visual indications of bloom formation and growth may be evaluated by field instrument scans of levels of chlorophyll and phycocyanin. Visual and field analytical indications of bloom formation or expansion may then be further assessed by laboratory phytoplankton identification and counts of cyanobacteria. Elevated cyanobacteria abundances may trigger subsequent testing for and quantification of cyanotoxins.
- Planning for contingencies: Have plans in place that address questions like:
- What active remedies will be considered if MNA ceases to be viable?
- Is funding in place in case an alternative to MNA should be implemented?
- Have vendors, suppliers, or other resources been identified if active treatment becomes necessary?
EFFECTIVENESS
- Any water body type
- Any surface area or depth
- Any mixing regime
- Any water body use
- Confined to bloom area
- Dissipation may occur through natural cycles
NATURE OF HCB
- Surface or subsurface HCBs
- Toxic and nontoxic HCBs
- Almost any area except perhaps a drinking water source
- Intervention strategy
ADVANTAGES
- Low cost relative to active, engineered remedies
- No expertise, infrastructure, or special equipment required
- No chemical additives or physical manipulations
- No wastes or by-products
LIMITATIONS
- May or may not yield a bloom decline (Van den Wyngaert et al. 2011)
- Substantial staff time for signage, outreach, and monitoring
- Requires outreach to local residents and lake users for threat, aesthetics of the water, and recreational limits
- Untreated nascent or resident cyanobacteria populations may re-seed the water body (Preston, Stewart, and Reynolds 1980)
- Recurrent monitoring is often needed to reassess risks
COST ANALYSIS
Relative cost per growing season: MNA
| ITEM | RELATIVE COST PER GROWING SEASON |
| Labor | $ |
| O&M Costs | $ |
| Occasional Monitoring | $ |
| OVERALL | $ |
The primary costs are for producing and distributing outreach materials, signage for local water body users, labor for posting and removing signs, and labor for monitoring water quality conditions. In addition to monitoring during an active bloom event, some monitoring should also be considered to document bloom dissipation or persistence.
REGULATORY AND POLICY CONSIDERATIONS
If the water body is a public water supply source, the municipal authority or water purveyor may need to actively treat the HCB rather than take the MNA approach. For treatment, regulatory approvals or permits may be needed. However, careful consideration and planning should precede selecting MNA, including soliciting input from stakeholders and securing public consensus.
REFERENCES
Preston, T., W. D. P. Stewart, and C. S. Reynolds. 1980. “Bloom-forming cyanobacterium Microcystis aeruginosa overwinters on sediment surface.” Nature 288 (5789):365-367. doi: 10.1038/288365a0.
Stevens, D. K., and R. I. Krieger. 1991. “Stability studies on the cyanobacterial nicotinic alkaloid anatoxin-A.” Toxicon 29 (2):167-79. doi: 10.1016/0041-0101(91)90101-v.
USEPA. 2019. “Recommendations for Cyanobacteria and Cyanotoxin Monitoring in Recreational Waters EPA 823-R-19-001.” https://www.epa.gov/sites/production/files/2019-09/documents/recommend-cyano-rec-water-2019-update.pdf.
Van den Wyngaert, Silke, Michaela M. Salcher, Jakob Pernthaler, Michael Zeder, and Thomas Posch. 2011. “Quantitative dominance of seasonally persistent filamentous cyanobacteria (Planktothrix rubescens) in the microbial assemblages of a temperate lake.” Limnology and Oceanography 56 (1):97-109. doi: 10.4319/lo.2011.56.1.0097.
Yamamoto, Yoshimasa, and Hiroyuki Nakahara. 2009. “Life Cycle of Cyanobacterium Aphanizomenon flos-aquae.” Taiwania 54. doi: 10.6165/tai.2009.54(2).113.
Zastepa, A., Z. E. Taranu, L. E. Kimpe, J. M. Blais, I. Gregory-Eaves, R. W. Zurawell, and F. R. Pick. 2017. “Reconstructing a long-term record of microcystins from the analysis of lake sediments.” Science of The Total Environment 579:893-901. doi: 10.1016/j.scitotenv.2016.10.211.
NANOPARTICLES (IRON-BASED)
In-lake Intervention Strategy
Limited/Emerging Supporting Field Data
Several studies were reviewed that focused on iron-based nanoparticles and their ability to adsorb cyanobacteria and degrade cyanotoxins through oxidative transformation. The technology is used in remediating and treating water, wastewater, and groundwater (Kharisov et al. 2012). No open-water case studies for HCB management were found. Zero-valent iron (nZVI) and bimetallic nanoparticles, such as iron-nickel (Fe-Ni) and iron-palladium (Fe-Pd), can degrade microcystin-LR (MC-LR) in drinking water treatment, with Fe-Pd showing the greatest degradation of MC-LR over the broadest pH range (~95% removal, Gao et al. 2016). This treatment has also been used for several other microcystin congeners and cylindrospermopsin. Other metallic or elemental compounds in some nanoparticles include titanium dioxide (Okupnik, Contardo-Jara, and Pflugmacher 2015), zinc oxide (Mahawar et al. 2018), polypyrroles (Hena et al. 2016), graphene and graphene oxide (Malina et al. 2019), copper-char (Li et al. 2019), silver (Duong et al. 2016), and silica (Xiong et al. 2017).
EFFECTIVENESS
- Unknown in any field application
NATURE OF HCB
- Effective at pH 7.0 for microcystin-LR, -LA, and -YR and at pH 9.0 for MC-RR and cylindrospermopsin
- Use is limited to drinking water
- Intervention strategy
ADVANTAGES
- Quick reaction time
- Readily adsorbs and destroys many contaminants, including cyanotoxins
- Some by-products promote flocculation
- Can use magnetic particles
- Possible reuse
LIMITATIONS
- No field applications
- nZVI has poor performance but is effective when paired with other metal ions
- May bind other compounds before cyanotoxins
- Unknown long-term environmental impact
- Reused particles only 30%–40% effective after eight uses
CASE STUDY EXAMPLES
Laboratory-scale: nZVI and bimetallic nanoparticles (Fe-Ni and Fe-Pd) have been used to degrade MC-LR in drinking water. Fe-Pd showed the greatest degradation of MC-LR (~95% removal) with the broadest pH range. Ni and Pd act as a catalyst for the degradation of MC-LR, whereas nZVI alone tends to readily form iron oxides and hydroxides in water, reducing its surface reactivity with MC-LR (Gao et al. 2016).
The highest adsorption rate for MC-LR, -LA, and -YR was at pH 7.0, whereas the highest rate for MC-RR and cylindrospermopsin was at pH 9.0. Removal from potable water can be done using magnetophoretic nanoparticles of polypyrrole. Adsorption capacity dropped to 30%–40% after reusing eight times. Polypyrrole/Fe3O4 had a high potential to remove cyanotoxins and could potentially be a cost-effective solution based on its reusability (Hena et al. 2016).
Adeleye et al. (2016) noted that there is still the likely persistence of some nanomaterials in the environment after use. They also suggest that research is needed to focus on predicting nanocomposite toxicity, so each new particle does not have to be tested individually.
Cost information is scarce due to the recent development of the technology and the limited commercialization of the products (Adeleye et al. 2016).
Relative cost per growing season: Nanoparticles (iron-based)
| ITEM | RELATIVE COST PER GROWING SEASON |
| Material | $–$$ |
| Personal Protective Equipment | Unknown |
| Equipment | Unknown |
| Machinery | Unknown |
| Tools | Unknown |
| Labor | Unknown |
| O&M Costs | Unknown |
| Other Costs | Unknown |
| OVERALL | >$$ |
REGULATORY AND POLICY CONSIDERATIONS
Long-term toxicity of nanoparticles in the environment is unknown, which may limit the scope of use or release into the environment. These materials are considered emerging contaminants by USEPA (2014). There are federal and local regulations based on intended use and application area.
REFERENCES
Adeleye, Adeyemi S., Jon R. Conway, Kendra Garner, Yuxiong Huang, Yiming Su, and Arturo A. Keller. 2016. “Engineered nanomaterials for water treatment and remediation: costs, benefits, and applicability.” Chemical Engineering Journal 286:640-662. doi: 10.1016/j.cej.2015.10.105.
Duong, Thi Thuy, Thanh Son Le, Thi Thu Huong Tran, Trung Kien Nguyen, Cuong Tu Ho, Trong Hien Dao, Thi Phuong Quynh Le, Hoai Chau Nguyen, Dinh Kim Dang, Thi Thu Huong Le, and Phuong Thu Ha. 2016. “Inhibition effect of engineered silver nanoparticles to bloom forming cyanobacteria.” Advances in Natural Sciences: Nanoscience and Nanotechnology 7 (3):035018. doi: 10.1088/2043-6262/7/3/035018.
Gao, Ying, Feifeng Wang, Yan Wu, Ravendra Naidu, and Zuliang Chen. 2016. “Comparison of degradation mechanisms of microcystin-LR using nanoscale zero-valent iron (nZVI) and bimetallic Fe/Ni and Fe/Pd nanoparticles.” Chemical Engineering Journal 285:459-466. doi: 10.1016/j.cej.2015.09.078.
Hena, S., R. Rozi, S. Tabassum, and A. Huda. 2016. “Simultaneous removal of potent cyanotoxins from water using magnetophoretic nanoparticle of polypyrrole: adsorption kinetic and isotherm study.” Environ Sci Pollut Res Int 23 (15):14868-80. doi: 10.1007/s11356-016-6540-5.
Kharisov, Boris I., H. V. Rasika Dias, Oxana V. Kharissova, Victor Manuel Jiménez-Pérez, Betsabee Olvera Pérez, and Blanca Muñoz Flores. 2012. “Iron-containing nanomaterials: synthesis, properties, and environmental applications.” RSC Advances 2 (25):9325-9358. doi: 10.1039/C2RA20812A.
Li, Ronghua, Hui Huang, Jim J. Wang, Wen Liang, Pengcheng Gao, Zengqiang Zhang, Ran Xiao, Baoyue Zhou, and Xiaofeng Zhang. 2019. “Conversion of Cu(II)-polluted biomass into an environmentally benign Cu nanoparticles-embedded biochar composite and its potential use on cyanobacteria inhibition.” Journal of Cleaner Production 216:25-32. doi: 10.1016/j.jclepro.2019.01.186.
Mahawar, Himanshu, Radha Prasanna, Shashi Bala Singh, and Lata Nain. 2018. “Influence of Silver, Zinc Oxide and Copper Oxide Nanoparticles on the Cyanobacterium Calothrix elenkinii.” BioNanoScience 8 (3):802-810. doi: 10.1007/s12668-018-0543-2.
Malina, Tomáš, Eliška Maršálková, Kateřina Holá, Jiří Tuček, Magdalena Scheibe, Radek Zbořil, and Blahoslav Maršálek. 2019. “Toxicity of graphene oxide against algae and cyanobacteria: Nanoblade-morphology-induced mechanical injury and self-protection mechanism.” Carbon 155:386-396. doi: 10.1016/j.carbon.2019.08.086.
Okupnik, Annette, Valeska Contardo-Jara, and Stephan Pflugmacher. 2015. “Potential role of engineered nanoparticles as contaminant carriers in aquatic ecosystems: Estimating sorption processes of the cyanobacterial toxin microcystin-LR by TiO2 nanoparticles.” Colloids and Surfaces A: Physicochemical and Engineering Aspects 481:460-467. doi: 10.1016/j.colsurfa.2015.06.013.
USEPA. 2014. “Technical Fact Sheet – Nanomaterials EPA 505-F-14-002 “. Washington, D. C.: U. S. Environmental Protection Agency, Office of Solid Waste and Emergency Response. https://19january2017snapshot.epa.gov/sites/production/files/2014-03/documents/ffrrofactsheet_emergingcontaminant_nanomaterials_jan2014_final.pdf.
Xiong, W., Y. Tang, C. Shao, Y. Zhao, B. Jin, T. Huang, Y. Miao, L. Shu, W. Ma, X. Xu, and R. Tang. 2017. “Prevention of cyanobacterial blooms using nanosilica: a biomineralization-inspired strategy.” Environ Sci Technol 51 (21):12717-12726. doi: 10.1021/acs.est.7b02985.
ORGANIC BIOCIDES
In-lake Prevention and Intervention Strategy
Limited/Emerging Supporting Field Data
Several research groups have explored the possibility of controlling cyanobacterial blooms using natural biocidal compounds or synthetic analogs. These compounds are not one group, or a derivate of a similar group or classification of molecules. Instead, they represent natural or synthetically modified extracts from various sources. By definition, a biocide is any compound (preservative, insecticide, disinfectant, pesticide, herbicide, fungicide, etc.) that is used for controlling a microorganism that is harmful to human or animal health (USEPA 2019). These organic biocides can range in algal and cyanobacterial targets, and there is an extensive literature of possible ecological end points. In some cases, it is not known how these compounds function; only observations of the effects (for example, algistatic or algaecidal and cyanostatic or cyanocidal) these biocides may have on target organisms are available. In some cases, compounds registered as biocides with USEPA for the control of cyanobacteria are used for limited instances, such as industrial cooling waters and biofouling, and not for surface recreational water bodies.
In general, organic biocides can be broken down into two categories: (1) those that are extracted from plants and (2) those that are natural derivatives of specific metabolites of other microorganisms or plants (NEIWPCC 2015). One potential example of a commonly used, known natural biocides are barley and rice straw extracts, which is expanded upon further in its own strategy.
EFFECTIVENESS
- Varies depending on the biocide and its application
NATURE OF HCB
- Since this is not a homogeneous group of compounds, the product will vary for the nature of each HCB. For USEPA-approved products, follow the application guidance for the nature of the HCB bloom experienced.
- Prevention and intervention strategy
Various natural compounds have been considered for their potential activity against cyanobacterial blooms and cyanotoxins, including:
- Barley straw and its extracts L-Lysine
- tellimagrandin II
- tryptamine
- nonanoic acid
- β-ionone
- geranyl acetone
While the above is not an exhaustive list of all natural biocidal compounds, several compounds have been examined (NEIWPCC 2015), generally in small-scale studies. Broader ecological impacts may not be known or fully understood. Exhaustive reviews of natural compounds, such as those conducted by Shao et al. (2013), note that many of these compounds may only be weakly cyanocidal or only exhibit inhibitory effects at very high concentrations. Additional concerns are that some organic biocide compounds can themselves be sources of nitrogen or phosphorus, important for additional algal or cyanobacterial growth. Use of some of these compounds, such as L-lysine, may enhance eutrophication by introducing exogenous sources of nitrogen.
ADVANTAGES
- Cost can be lower, depending on the organic biocide and the source, compared to chemical algaecides
- Some extracts can be prepared on site with minimal equipment
- Some natural compounds may degrade with no off-target effects noted
LIMITATIONS
- Limited documented application for all organic biocides as an intervention technique for HCBs
- Depending on mechanism of action, cyanotoxin release can occur
- Some risk of enhancing eutrophication in the use of several compounds
- Human and animal toxicity data are limited
- High purity extracts may be cost-prohibitive to effectively control blooms
COST ANALYSIS
Relative cost per growing season: Organic biocides
| ITEM | RELATIVE COST PER GROWING SEASON |
| Material | $–$$$ |
| Personal Protective Equipment | $–$$ |
| Equipment | $–$$ |
| Labor | $ |
| O&M Costs | $ |
| OVERALL | $$ |
Estimating cost is difficult for this technique due to the numerous variables. The cost and difficulty in generating the compound is a limiting factor, as is “growing” the source material. Some material, such as L-lysine, can be extracted in abundance at low cost. Others, as described in the literature, require several purification steps to isolate the targeted compound. In general, the simpler the extraction method, the lower the cost.
Some specialized equipment, such as sprayers or on-site grinders, may need to be purchased if the extract must be performed fresh.
CASE STUDY EXAMPLES
Dianch Lake, China: The cyanocidal effects of L-lysine and malonic acid were evaluated in enclosures with blooms of Microcystis aeruginosa (Kaya et al. 2005).
Three enclosures, measuring 10 m by 10 m by 1.3–1.5 m depth were established and monitored over 28 days. Enclosure A served as the control, B served as L-lysine alone, and C served as L-lysine + malonic acid.
Upon initial spraying, blooms resolved in both enclosures B and C; however, within 7 days a rebound bloom of M. aeruginosa appeared in enclosure B.
No rebound bloom was documented in enclosure C, and enhanced macrophyte growth was observed.
By the end of 28 days, no recovery of L-lysine or malonic acid could be detected, indicating that possible complete degradation of these compounds had occurred.
Some organic biocides already have USEPA registration. Additional products are registered as organic biocides, but only for application in specific environments. Some products, though naturally derived, have not been evaluated for short- or long-term toxicity in humans or other aquatic organisms and may pose a hazard. A “natural” or “organic” product is not necessarily safe and could have greater impacts on the ecosystem than the HCB it is purported to treat.
REFERENCES
Kaya, K., Y. D. Liu, Y. W. Shen, B. D. Xiao, and T. Sano. 2005. “Selective control of toxic Microcystis water blooms using lysine and malonic acid: an enclosure experiment.” Environ Toxicol 20 (2):170-8. doi: 10.1002/tox.20092.
NEIWPCC. 2015. “Harmful Algal Bloom Control Method Synopses.” New England Interstate Water Pollution Control Commission. https://www.neiwpcc.org/neiwpcc_docs/NEIWPCC_HABControlMethodsSynopses_June2015.pdf.
Shao, J., R. Li, J. E. Lepo, and J. D. Gu. 2013. “Potential for control of harmful cyanobacterial blooms using biologically derived substances: problems and prospects.” J Environ Manage 125:149-55. doi: 10.1016/j.jenvman.2013.04.001.
USEPA. 2019. “I-BEAM Glossary of Terms.” U. S. Environmental Protection Agency. https://ofmpub.epa.gov/sor_internet/registry/termreg/searchandretrieve/glossariesandkeywordlists/search.do?details=&vocabName=I-BEAM%20Glossary%20of%20Terms.
OZONATION
In-lake Intervention Strategy
Limited Supporting Field Data
Ozonation is an advanced oxidation technique that works by infusing ozone gas into water. There is a long history of process-level ozonation use to disinfect drinking water and wastewater (Loeb et al. 2012, Rice 1991). Ozone attacks the chemical bonds within cyanotoxins and other compounds, leading to rapid degradation. Ozone treatment requires on-site generation of ozone gas, due to a short half-life of the compound. In general, ozone is produced by passing purified air through an electric discharge to convert oxygen to ozone. Ozone is not readily soluble in water, particularly compared to other oxidative compounds such as chlorine, so it requires a delivery mechanism such as a diffuser for application. Application methods vary but do require on-site infrastructure for application. Ozonation has substantial documentation for applications in both drinking water and wastewater processing and ozone nanobubbles have been used in ponds, lakes, and bays to reduce planktonic chlorophyll concentrations (for example, NBS 2018). It is also a fourth step in the surfactant-flotation-skimming-ozonation technique described in the skimming and harvesting strategy.
Except for non-replicated ozone nanobubble projects in Asia and Florida, ozonation is still a research technique for the treatment of HCBs in surface waters, with no current peer-reviewed literature on surface water treatment. Ozonation has been shown to oxidize multiple cyanotoxin classes (Newcombe and Nicholson 2004). For example, pilot and laboratory work suggest that significant reductions in microcystin concentrations can be achieved with an ozone concentration of at least 0.3 mg/L and a contact time of at least 5 minutes. Similar results were also observed for anatoxin-a at somewhat higher ozone concentrations (Newcombe and Nicholson 2004). The amount of dissolved organic carbon in the water strongly affects the efficacy of ozone treatment on cyanotoxins (Staehelin and Hoigne 1985). Ozonation also shows promise in lysing HCB organisms directly and has been shown experimentally to lyse cells of several genera, including Microcystis spp., Dolichospermum spp., Aphanizomenon spp., and Pseudanabaena spp. (Pandhal et al. 2018, Zamyadi et al. 2015).
EFFECTIVENESS
- Water body type: Pond, lake/reservoir
- Any surface area or depth
- Any trophic state
- Any mixing regime
- Water body uses: Drinking water, treated wastewater/effluent
NATURE OF HCB
- Shown as useful in drinking water treatment for reservoirs and other source waters with chronic blooms
- Can kill Microcystis spp. and other cells with sufficient contact time
- Intervention strategy
ADVANTAGES
- Ozone treatment in benchtop applications has been shown to be capable of completely oxidizing multiple cyanotoxin classes, including microcystins, anatoxin-a, and cylindrospermopsin. However, it has not been shown to oxidize saxitoxins efficiently (Cheng et al. 2009, Fawell et al. 1993, Newcombe and Nicholson 2004, Onstad et al. 2007, Rositano et al. 2001).
- Ozonation can also lyse cells, with the effectiveness depending on the ozone concentration and contact time; with toxin oxidation noted above, ozonation is a possible broadly applicable technique.
- Ozone also removes many other water impurities, including taste and odor compounds (Ho, Newcombe, and Croué 2002), Cryptosporidium, and multiple organic compounds.
LIMITATIONS
- Ozone treatment is likely not suitable for a one-time application, as it must be generated on-site.
- Ozone treatment has an extremely high oxidation potential and is non-selective in the organisms that are killed (both HCB and non-HCB organisms).
- Ozone treatment generally results in cell lysis, which could release cyanotoxins contained within HCB cells.
- The effectiveness of ozone treatment is impacted by the concentration of organic matter in the system; therefore, it may require pretreatment if organic matter loads are high.
- Ozone treatment does not leave residuals; therefore, treatment is short lived and requires reapplication.
- If the water’s metal content is high, ozonation will form insoluble metal oxides that would potentially need to be removed.
- Applicator protection may be required.
Treatment of HCB events in surface water via ozonation is still in development. This technique has been applied in several field situations via dispersal of ozone nanobubbles to reduce planktonic chlorophyll concentrations (NBS 2018, without any species information however). Ozonation remains an emerging strategy, as it is still largely a research technique.
CASE STUDY EXAMPLES
Laboratory-scale: Pandhal et al. (2018) conducted a benchtop study using a novel ozone generation and application method. The study used a low-temperature plasma dielectric barrier discharge reactor and a fluidic oscillator diffuser, which has lower energy requirements than other systems. Together, this method delivers ozone in microbubbles, which increases the solubility of ozone and therefore increases the contact time.
This study showed that microbubble delivery of ozone via this system rapidly degrades microcystins, with complete oxidation of MC-LR in 2 minutes at an ozone flow rate of 1 L/min. Importantly, the treatment showed a large decrease in toxicity of the microcystin, with the microcystin by-products showing a substantial decrease in inhibitory activity. Lysis of Microcystis aeruginosa cells was observed within 20 minutes.
Alternative ozone generation and delivery technologies such as described in Pandhal et al. (2018) have the potential to lower the operation costs of ozonation, making the treatment more affordable in the future.
COST ANALYSIS
Large-scale ozonation use is estimated to be the most expensive of the advanced oxidation processes, according to a cost analysis conducted by Dore et al. (2013) for smaller systems. Primary expenses are capital costs, which can be in the millions, and yearly operational costs, which can be in the hundreds of millions. Dore et al. (2013) estimated that ozone treatment could cost between $0.10 and $0.50/m3 water, with costs decreasing precipitously at treatment volumes >10,000 m3/d.
Relative cost per growing season: Ozonation
| ITEM | RELATIVE COST PER GROWING SEASON |
| Material | $$$ |
| Personal Protective Equipment | $$ |
| Equipment | $$$ |
| Machinery | $$$ |
| Tools | $$$ |
| Labor | $$$ |
| O&M Costs | $$$ |
| Delivery | $$$ |
| OVERALL | $$$ |
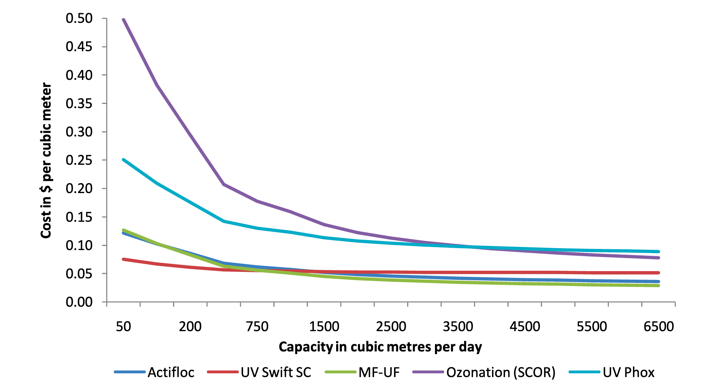
Figure C-9. Cost reductions vs. treated volumes for several remediation strategies.
Source: Dore et al. (2013). Used with permission.
REGULATORY AND POLICY CONSIDERATIONS
Use of ozonation at the process level requires an investment in infrastructure, but the technique is already used in many cities throughout the United States in drinking water and wastewater treatment plants. Use of ozonation has been accepted in these applications for many decades (Loeb et al. 2012). Ozonation for treatment of active HCB events in surface waters might be feasible in the future (for example, via nanobubbles), but at present it remains a research technique. Excess ozone will naturally convert to oxygen, although at very high concentrations ozone can damage fish gills. With ozone monitoring, ecosystem impacts of treated water can be minimized, likely increasing public acceptance of the method compared to chemical applications and their residuals. Human exposure to high ozone levels should be avoided and permits for its use should be explored.
REFERENCES
Cheng, X., H. Shi, C. D. Adams, T. Timmons, and Y. Ma. 2009. “Effects of oxidative and physical treatments on inactivation of Cylindrospermopsis raciborskii and removal of cylindrospermopsin.” Water Sci Technol 60 (3):689-97. doi: 10.2166/wst.2009.385.
Dore, Mohammaed H., Rajiv G. Singh, Arian Khaleghi-Moghadam, and Gopal Achari. 2013. “Cost differentials and scale for newer water treatment technologies.” International Journal of Water Resources ad environmental Engineering 5 (2):100-109.
Fawell, JK, J Hart, HA James, and W Parr. 1993. “Blue-green algae and their toxins-analysis, treatment and environmental control.” Water Supply 11 (3/4):109-115.
Ho, L., G. Newcombe, and J. P. Croué. 2002. “Influence of the character of NOM on the ozonation of MIB and geosmin.” Water Res 36 (3):511-8. doi: 10.1016/s0043-1354(01)00253-6.
Loeb, Barry L., Craig M. Thompson, Joseph Drago, Hirofumi Takahara, and Sylvie Baig. 2012. “Worldwide ozone capacity for treatment of drinking water and wastewater: a review.” Ozone: Science & Engineering 34 (1):64-77. doi: 10.1080/01919512.2012.640251.
NBS, (Nanobubble Solutions LImited). 2018. Project to Eliminate Blue-Green Algae at the Outer Moat of the Imperial Palace Hibiya, Tokyo: Project Overview Japan.
Newcombe, Gayle, and B. C. Nicholson. 2004. “Water treatment options for dissolved cyanotoxins.” Journal of Water Supply: Research and Technology – AQUA 53:227-239. doi: 10.2166/aqua.2004.0019.
Onstad, G. D., S. Strauch, J. Meriluoto, G. A. Codd, and U. Von Gunten. 2007. “Selective oxidation of key functional groups in cyanotoxins during drinking water ozonation.” Environ Sci Technol 41 (12):4397-404. doi: 10.1021/es0625327.
Pandhal, J., A. Siswanto, D. Kuvshinov, W. B. Zimmerman, L. Lawton, and C. Edwards. 2018. “Cell lysis and detoxification of cyanotoxins using a novel combination of microbubble generation and plasma microreactor technology for ozonation.” Front Microbiol 9:678. doi: 10.3389/fmicb.2018.00678.
Rice, R. G. 1991. “Recent advances in ozone treatment of drinking water.” In Chemistry for the Protection of the Environment, edited by L. Pawlowski, W. J. Lacy and J. J. Dlugosz, 713-730. Boston, MA: Springer US.
Rositano, J., G. Newcombe, B. Nicholson, and P. Sztajnbok. 2001. “Ozonation of NOM and algal toxins in four treated waters.” Water Res 35 (1):23-32. doi: 10.1016/s0043-1354(00)00252-9.
Staehelin, Johannes, and Juerg Hoigne. 1985. “Decomposition of ozone in water in the presence of organic solutes acting as promoters and inhibitors of radical chain reactions.” Environmental Science & Technology 19 (12):1206-1213. doi: 10.1021/es00142a012.
Zamyadi, A., L. A. Coral, B. Barbeau, S. Dorner, F. R. Lapolli, and M. Prévost. 2015. “Fate of toxic cyanobacterial genera from natural bloom events during ozonation.” Water Res 73:204-15. doi: 10.1016/j.watres.2015.01.029.
PHOSPHORUS-BINDING COMPOUNDS
In-lake Prevention Strategy
Substantial Supporting Field Data
Geoengineering involves the addition of phosphorus-binding (P-binding) elements to lake bottom sediments to bind sediment phosphorus and control the release of phosphorus from sediment during low-oxygen conditions (for example, internal phosphorus loading control). Minerals containing aluminum have been employed for decades and are naturally abundant in the environment; aluminum content of soils and sediment is generally between 1% and 10% (Sorenson et al. 1974, USGS 1984). Natural lake water concentrations of aluminum range from 10 µg/L to100 µg/L (Wetzel 2001). Aluminum acts by converting iron-bound and pore water phosphorus to aluminum-bound phosphorus. Aluminum-bound phosphorus is stable under low-oxygen conditions and a wide pH range and is thus considered permanently inactivated after treatment. Aluminum is commonly applied as alum, sodium aluminate, and polyaluminum chloride (alum and sodium aluminate are applied together as a buffer in low-alkalinity waters). The material is typically applied as a liquid (by a treatment barge) to the lake surface, where it forms a floc that then settles to the bottom and is incorporated into the sediment. In some cases, a solid aluminum salt is applied. It is one of the few strategies used for internal phosphorus control that has extensive field evidence, and it is the only geoengineering material that has extensive field evidence of longevity, effectiveness, and safety.
Several factors determine the effectiveness and longevity of aluminum treatment. For example, aluminum treatment will be more effective for lakes with significant internal phosphorus loading relative to watershed phosphorus loads. A high lake surface to tributary watershed area ratio increases treatment longevity, as a larger ratio is generally indicative of a lower flushing rate. An approximate cutoff is a 7.2 ratio of watershed to lake area (see Huser et al. 2016) for factors that may affect treatment longevity). Accurate aluminum dosing also increases effectiveness and longevity (Huser and Pilgrim 2014, Pilgrim, Huser, and Brezonik 2007). Longevity and effectiveness are greater for lakes with stable thermocline and infrequent mixing. However, this does not preclude the use of aluminum for systems that mix, given that phosphorus transport from the lake bottom is mediated by mixing. Aluminum may be very effective for systems that mix, but longevity of a treatment is largely determined by new phosphorus inputs. Shallow systems experience more phosphorus load per unit lake volume compared to a deep lake, given equal watershed inputs. Although longevity is often reduced for shallow systems primarily due to continued excess external loading of phosphorus, treatment can be used effectively for shallow lakes if factors such as benthic feeding fish, invasive aquatic plants, and best management practices (ponds, wetlands, and filtration for phosphorus removal) are also implemented (Bartodziej, Blood, and Pilgrim 2017). Trophic state is not a determinant of use, and aluminum can be used for a wide range of sediment phosphorus concentrations. For eutrophic systems, application is most often conducted in the spring and fall to avoid algal blooms or aquatic plants interfering with floc formation and settling. It is also notable that treatment effectiveness has been recently demonstrated for estuarine waters using PAC (Rydin et al. 2017).
EFFECTIVENESS
- Water body types: Pond, lake/reservoir
- Any surface area: high ratio of lake surface to tributary watershed area increases longevity
- Any depth:effectiveness in shallow systems depends on other factors (benthic feeding fish, invasive aquatic plants) and implementing best management practices
- Any trophic state: aluminum can be used for a wide range of sediment phosphorus concentrations when best management practices are also implemented
- Any mixing regime
- Any water body use; see product label for specifications on water body use
- Greater effectiveness for lakes with high internal versus external phosphorus loading
- Greater effectiveness and longevity when using aluminum dosing methods based on iron-bound phosphorus concentration in the sediment
NATURE OF HCB
- Most planktonic HCB types
- Toxic and nontoxic HCBs
- HCBs that are primarily phosphorus limited
- HCBs induced by mid-summer internal phosphorus loading and lake mixing
- Prevention strategy

Figure C-10. Applying P-binding compounds to a lake.
Source: K. Pilgrim, Barr Engineering Company. Used with permission.
Alternative methods include other P-binding material additions, such as modified bentonite clay (Robb et al. 2003), lime (Ca[OH]2, CaCO3), and ferric chloride (FeCl3) (Chorus and Bartram 1999). Note, however, that Triest, Stiers, and Van Onsem (2016) caution the use of lime, as lime-induced increases in lake pH (>8.0) selects for cyanobacteria. Implementation of other strategies in the watershed can reduce incoming phosphorus (see Section 7), thereby making in-lake P-binding additions more effective and reducing need for reapplication.
ADVANTAGES
- Experienced application contractors available in the United States
- Relatively low cost
- Adverse effects understood and can be controlled to avoid effects on aquatic life
- Can reduce phosphorus during critical summer months when HCB potential is elevated
- Unlike iron, aluminum minerals are stable and not released under anerobic conditions
- During treatment, flocculation is rapid, and aluminum floc does not reside for an extended period in the water column
- Water quality criteria have been developed by USEPA (2018); hence, potential risk levels for aquatic life have been quantified
LIMITATIONS
- Effectiveness may be reduced for small water bodies with large watersheds
- Large sediment and phosphorus load from the watershed can limit treatment longevity and require reapplication
- Permitting is state specific; some states are more accustomed to and accepting of aluminum treatment than others
- Application may be impractical for very large lakes
- Buffered aluminum forms or bases with aluminum should be used for low-alkalinity waters
- Phosphorus monitoring through time should follow aluminum additions to identify if/when phosphorus binding is saturated and another addition should be considered
CASE STUDY EXAMPLES
Step 1. Identify the depth of elevated mobile phosphorus in sediment.
Step 2. Calculate the average concentration of mobile phosphorus in the sediment with elevated phosphorus.
Step 3. Identify the aluminum dose necessary to reduce mobile phosphorus to the desired level. This may reduce mobile phosphorus as much as possible or reduce it to background levels identified by sediment cores. Use the methods of Pilgrim, Huser, and Brezonik (2007) to identify the expected mobile phosphorus reduction. An example of this relationship is shown below.
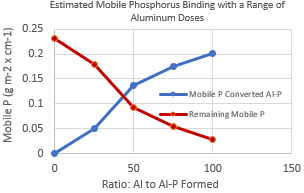
Step 4. Identify the depth of sediment to be treated and calculate the total dose to be applied to the lake as g aluminum/sq. m of lake bottom sediment (Huser and Pilgrim 2014, Pilgrim, Huser, and Brezonik 2007).
COST ANALYSIS
Overall, aluminum treatment is considered to be cost-effective compared to other phosphorus control methods (Bartodziej, Blood, and Pilgrim 2017). The cost to apply aluminum by commercial applicators is typically quoted as per gallon applied. The cost per gallon applied is the market cost of the liquid aluminum product delivered to the site, plus a markup by the contractor to apply the product. The amount of aluminum needed is often determined by an aerial dose quoted as grams of aluminum per square meter of lake bottom area. This aerial dose is determined by the amount of phosphorus in the lake bottom sediment. More phosphorus (often called mobile phosphorus) in the lake bottom sediments requires more aluminum. The initial application is expensive, but aluminum treatments can be expected to last 11 years, resulting in a low average cost per growing season. The cost will need to be determined on a site-specific basis. Previous estimates from 14 studies indicate an average cost of $5,275/acre in U.S. dollars (Appendix C.2).
Relative cost per growing season: P-binding compounds
| ITEM | RELATIVE COST PER GROWING SEASON |
| Material | $$ |
| Personal Protective Equipment | $ |
| Equipment | $$$ |
| Machinery | $$ |
| Tools | $ |
| Labor | $$ |
| O&M Costs | $ |
| OVERALL | $–$$ |
REGULATORY AND POLICY CONSIDERATIONS
You can expect a large range of comfort with this prevention approach, and each state will have a different aluminum treatment policy. The approval process may not be straightforward, as aluminum treatment does not fall under the jurisdiction of most established permitting programs. You will need to contact the state limnologist or appropriate permitting staff.
The primary regulatory hurdle with this method is the potential for aluminum-induced, short-term aquatic toxicity during application. Long-term effects (chronic toxicity) have not been observed for typically applied doses (Clearwater, Hickey, and Thompson 2014). The literature regarding aluminum aquatic toxicity is extensive and largely resulting from acid rain research in the 1970s and 1980s (see USEPA 2018 for an extensive review of aluminum toxicity). However, conditions in eutrophic lakes are very different compared to acidified lakes, considering the predominant condition where aluminum is acutely toxic (pH<5.5). The pH range in eutrophic lakes is most often in the neutral range (pH 7.0–9.0), and there is a large body of evidence demonstrating safe application of aluminum for phosphorus treatment, even in low-alkalinity waters. Numerous studies have shown that aluminum treatments had no adverse effect on aquatic life. In some cases, fish or benthic invertebrate abundances increased (Buergel and Soltero 1983, Glilman 2006, Narf 1985, Narf 1990, Smeltzer 1990). In contrast to acidification-related aluminum studies, water column aluminum concentrations have been shown to decrease in the water after the floc has settled to the sediment (Pilgrim and Huser unpublished). This is due to the reduction of particulate matter in the water (algae) that can bind with natural aluminum entering the water body. The increasingly common use of buffered alum and sodium aluminate treatment has improved the ability to regulate pH during treatment, and application during spring and fall has avoided potential complications with phytoplankton and cyanobacterial blooms.
REFERENCES
Bartodziej, W. M., S. L. Blood, and Keith Pilgrim. 2017. “Aquatic plant harvesting: An economical phosphorus removal tool in an urban shallow lake.” Journal of Aquatic Plant Management 55:26-34.
Buergel, Pamela M., and Raymond A. Soltero. 1983. “The distribution and accumulation of aluminum in rainbow trout following a whole-lake alum treatment.” Journal of Freshwater Ecology 2 (1):37-44. doi: 10.1080/02705060.1983.9664574.
Chorus, Ingrid, and Jamie Bartram. 1999. “Toxic cyanobacteria in water : a guide to their public health consequences, monitoring and management.” Geneva: World Health Organization. http://www.toxicologia.org.ar/wp-content/uploads/2016/03/toxcyanobacteria.pdf.
Clearwater, Susan, Christopher Hickey, and Karen Thompson. 2014. “The effect of chronic exposure to phosphorus-inactivation agents on freshwater biota.” Hydrobiologia 728. doi: 10.1007/s10750-014-1805-9.
Glilman, Bruce. 2006. “Pre- and Post-Alum Treatment Survey of Honeoye Lake Macrobenthos: Comprehensive Field Inventory and Data Summary July 2005 and November 2006.” Department of Environmental Conservation and Horticulture, Finger Lakes Community College. https://hvaweb.org/Resources/Documents/Pre%20and%20Post-alum%20Survey%20of%20Honeoye%20Lake%20final.pdf.
Huser, B. J., S. Egemose, H. Harper, M. Hupfer, H. Jensen, K. M. Pilgrim, K. Reitzel, E. Rydin, and M. Futter. 2016. “Longevity and effectiveness of aluminum addition to reduce sediment phosphorus release and restore lake water quality.” Water Res 97:122-32. doi: 10.1016/j.watres.2015.06.051.
Huser, B. J., and K. M. Pilgrim. 2014. “A simple model for predicting aluminum bound phosphorus formation and internal loading reduction in lakes after aluminum addition to lake sediment.” Water Res 53:378-85. doi: 10.1016/j.watres.2014.01.062.
Narf, R. P. 1985. “Impact of Phosphorus Reduction via Metalimnetic Alum Injection in Bullhead Lake, Wisconsin.” Madison, WI. https://dnr.wi.gov/files/PDF/pubs/ss/SS0153.pdf.
Narf, Richard P. 1990. “Interactions of Chironomidae and Chaoboridae (Diptera) with Aluminum Sulfate Treated Lake Sediments.” Lake and Reservoir Management 6 (1):33-42. doi: 10.1080/07438149009354693.
Pilgrim, K. M., and B. J. Huser. unpublished. “Unpublished data from Minnesota and Sweden.”
Pilgrim, K. M., B. J. Huser, and P. L. Brezonik. 2007. “A method for comparative evaluation of whole-lake and inflow alum treatment.” Water Res 41 (6):1215-24. doi: 10.1016/j.watres.2006.12.025.
Robb, Malcolm, Bruce Greenop, Zoë Goss, Grant Douglas, and John Adeney. 2003. “Application of Phoslock™, an innovative phosphorus binding clay, to two western australian waterways: preliminary findings.” Hydrobiologia 494. doi: 10.1023/A:1025478618611.
Rydin, E., L. Kumblad, F. Wulff, and P. Larsson. 2017. “Remediation of a Eutrophic Bay in the Baltic Sea.” Environ Sci Technol 51 (8):4559-4566. doi: 10.1021/acs.est.6b06187.
Smeltzer, Eric. 1990. “A successful alum/aluminate treatment of Lake Morey, Vermont.” Lake and Reservoir Management 6 (1):9-19. doi: 10.1080/07438149009354691.
Sorenson, J. R., I. R. Campbell, L. B. Tepper, and R. D. Lingg. 1974. “Aluminum in the environment and human health.” Environ Health Perspect 8:3-95. doi: 10.1289/ehp.7483.
Triest, Ludwig, Iris Stiers, and Stijn Van Onsem. 2016. “Biomanipulation as a nature-based solution to reduce cyanobacterial blooms.” Aquatic Ecology 50 (3):461-483. doi: 10.1007/s10452-015-9548-x.
USEPA. 2018. “Final Aquatic Life Ambient Water Quality Criteria for Aluminum 2018 EPA 822-R-18-001.” Washington, D. C.: U. S. Environmental Protection Agency, Office of Water. https://www.epa.gov/sites/production/files/2018-12/documents/aluminum-final-national-recommended-awqc.pdf.
USGS. 1984. “Element Concentrations in Soils and Other Surficial Materials of the Conterminous United States, Geological Survey Professional Paper 1270.” Alexandria, VA: U. S. Geological Survey. https://pubs.usgs.gov/pp/1270/.
Wetzel, Robert G. 2001. Limnology: Lake and River Ecosystems. San Diego, CA: Academic Press.
PEROXIDE APPLICATION
In-lake Intervention Strategy
Substantial Supporting Field Data
Substantial field evidence indicates that applying a crystalized peroxide compound or a liquid peroxide mixture to a non-flowing water body can rapidly reduce HCBs and cyanotoxins (Mattheiss, Sellner, and Ferrier 2017, Matthijs et al. 2012). The crystal can be deployed in several hours to a day. Two crystal types are now available: (1) one that sinks to the bottom for control of planktonic cyanobacteria in the water column, as well as near-bottom or bottom populations, and (2) one that is a floating, slowly dissolving particle that moves with surface blooms, responding to wind or other concentrating mechanisms. Field evidence from the Netherlands indicates that a lake-water-diluted peroxide solution can be effective in HCB control via dispersal at multiple depths (Matthijs et al. 2012). Effective peroxide concentrations appear to be 2.3 mg/L for Planktothrix agardhii, 3–4 mg/L for Aphanizomenon and Anabaena/Dolichospermum, and >5 mg/L for Microcystis aeruginosa. M. aeruginosa may require more than 5 mg/L, but zooplankton mortalities can occur much beyond 5 mg/L (Matthijs et al. 2016, Zhou et al. 2018).
EFFECTIVENESS
- Water body types: Pond, lake/reservoir, any non-flowing freshwater system
- Surface area: Small
- Depth: Shallow
- Any trophic state
- Any mixing regime
- Any water body use
NATURE OF HCB
- All HCB types; planktonic, near-bottom, and bottom cyanobacteria
- Singular or repeating blooms
- Toxic or nontoxic HCBs
- Effective for most cyanobacteria
- Intervention strategy
ADVANTAGES
- Rapidly decomposes to O2 and H2O
- Oxidizes cyanobacterial cells and cyanotoxins
- Effective at <5 mg/L
- Modest cost per acre, with dose dependent on cyanobacterial biomass
- Field use common
LIMITATIONS
- Requires access to surface area (for example, a boat)
- Peroxide compounds need special handling and possible state-required training and application permit
- Can release toxins from cells (but peroxides can quickly oxidize these compounds)
- At H2O2 >5 mg/L, may impact zooplankton and fish
- May be less effective in highly turbid systems

Figure C-11. Granular and liquid peroxide application.
Source: J. Mattheiss, Hood CCWS, and Matthijs et al. (2012). Used with permission.
CASE STUDY EXAMPLES
Lake Anita Louise, Frederick County, Maryland, United States: Mattheiss, Sellner, and Ferrier (2017) reported that 350 pounds of peroxide crystals were dispersed over ~4.5 acres in a 10–12-foot-deep system from a small boat in approximately 3 hours. Peroxide concentrations approximated 3 mg/L and rapidly declined to background levels in 3 days. Densities of a P. agardhii surface bloom were dramatically reduced and remain low 4 years after treatment.
Various locations: Liquid application with peroxide levels at ~3 mg/L have also proved effective in Lake Koetshuis (Matthijs et al. 2012); Ouwerkerkse Kreek, Netherlands (Burson et al. 2014); and an Alabama aquaculture pond (Yang et al. 2018).
COST ANALYSIS
Costs for granule application are modest to moderate. Granules are used most often on ponds and small lakes, depending on the amount of the HCB and water body size. Liquid dosing is more expensive. Dosing and cost per acre are listed on each product, but seeking <5 mg/L in-lake H2O2 should be the goal. Granular peroxide compounds are not inexpensive, but cost is modest relative to mechanical strategies. However, one or two treatments per year or over several years may be required. Small boats with two people can disperse granular compounds, but special liquid-dispensing equipment (an additional cost) may be needed for multiple depth injections. Other cost estimates are presented in Appendix C.2.
Relative cost per growing season: Peroxide application
| ITEM | RELATIVE COST PER GROWING SEASON |
| Material | $–$$ |
| Personal Protective Equipment | $ |
| Equipment | $–$$$ |
| Labor | $–$$ |
| OVERALL | $$ |
REGULATORY AND POLICY CONSIDERATIONS
Applicator training and permits for application may be required in many states. Check individual state regulations.
REFERENCES
Burson, A., H. C. Matthijs, W. de Bruijne, R. Talens, R. Hoogenboom, A. Gerssen, P. M. Visser, M. Stomp, K. Steur, Y. van Scheppingen, and J. Huisman. 2014. “Termination of a toxic Alexandrium bloom with hydrogen peroxide.” Harmful Algae 31:125-135. doi: 10.1016/j.hal.2013.10.017.
Mattheiss, J, K. G. Sellner, and D. Ferrier. 2017. “Lake Anita Louise Peroxide Treatment Summary December 2016.” https://www.lakelinganore.org/wp-content/uploads/2017/01/Peroxide-Application-Summary-Report-Final.pdf.
Matthijs, H. C., P. M. Visser, B. Reeze, J. Meeuse, P. C. Slot, G. Wijn, R. Talens, and J. Huisman. 2012. “Selective suppression of harmful cyanobacteria in an entire lake with hydrogen peroxide.” Water Res 46 (5):1460-72. doi: 10.1016/j.watres.2011.11.016.
Matthijs, Hans C. P., Daniel Jančula, Petra M. Visser, and Blahoslav Maršálek. 2016. “Existing and emerging cyanocidal compounds: new perspectives for cyanobacterial bloom mitigation.” Aquatic Ecology 50 (3):443-460. doi: 10.1007/s10452-016-9577-0.
Yang, Zhen, Riley P. Buley, Edna G. Fernandez-Figueroa, Mario U. G. Barros, Soorya Rajendran, and Alan E. Wilson. 2018. “Hydrogen peroxide treatment promotes chlorophytes over toxic cyanobacteria in a hyper-eutrophic aquaculture pond.” Environmental Pollution 240:590-598. doi: 10.1016/j.envpol.2018.05.012.
Zhou, Q., L. Li, L. Huang, L. Guo, and L. Song. 2018. “Combining hydrogen peroxide addition with sunlight regulation to control algal blooms.” Environ Sci Pollut Res Int 25 (3):2239-2247. doi: 10.1007/s11356-017-0659-x.
SHADING WITH DYES (LIGHT FILTERING)
In-lake Prevention Strategy
Limited Supporting Field Data
Dyes may be added to ponds and small lakes to physically filter sunlight with the goal of reducing photosynthesis and cyanobacteria growth. A commercial dye product is added to the shoreline of ponds or small lakes beginning in spring and periodically during the growing season to reduce the potential for and severity of HCBs. These nontoxic dyes naturally disperse and can filter out certain light spectra, reducing light penetration and shading the water body. Dyes are available in blue, black, and other colors. Testing suggests that dyes are likely to be most effective on aquatic plants, algae, and cyanobacteria at least 2 feet below the surface (NYSFOLA 2009). Commercial dyes for this application have been available in the marketplace for decades, but there is limited published scientific demonstration of their effectiveness.
Application rates will vary by dye manufacturer, but dosing rates of commonly used dyes are in the range of 1–2 gallons of dye solution per million gallons of water (Madsen et al. 1999). After initial dye dosing, periodic re-doses are necessary to maintain the shade color and light-filtering properties and counter dye fading and dilution from inflowing water (Ludwig, Perschbacher, and Edziyie 2010).
If the pond or small lake is deeper than 2 feet and has a history of repeated cyanobacterial blooms, the dye light filtering and shading approach may be a prevention technology for you to consider, either alone or in conjunction with other technologies. Method practicality and costs largely hinge on the volume of the water body and the dilution caused by clear-water inflows from streams, springs, etc.; the larger the volume and dilution, the more dye you will need to add. While eutrophic waters are the most likely candidates for the approach, there are no established specific trophic state or mixing regime requirements. Using dye shading to limit photosynthesis may affect growth of some cyanobacterial species more than others, depending on light sensitivity and where they reside relative to the water surface. As a result, you may change the species of algae and cyanobacteria that predominate (NYSFOLA 2009, Suski et al. 2018).
Floating plastic balls have been suggested as another shading option, but they have not been used in HCB control (see Abridged Strategies).
EFFECTIVENESS
- Water body types: Pond, lake/reservoir
- Surface area: Small
- Any depth
- Trophic state: Eutrophic
- Any mixing regime
- Water body uses: Recreation, drinking water
NATURE OF HCB
- Subsurface HCBs
- Toxic and nontoxic HCBs
- Prevention strategy
ADVANTAGES
- Unlikely carry-over after bloom dissipation
- Low potential for adverse impacts
- Available and relatively inexpensive
- Minimal technical expertise, manpower, electricity, or specialized equipment needed
- Shading dyes appear to be nontoxic (USEPA 2005, WSDE 2016)
LIMITATIONS
- Cost-effective only for small lakes and those with long residence time
- Inhibits photosynthesis of all algae, not just cyanobacteria
- Can interfere with pigment analyses used to characterize blooms (Buglewicz and Hergenrader 1977)
- May alter lake ecology, changing dominant plant, algae, and fish species (NYSFOLA 2009, Suski et al. 2018)
- Limited proof of effectiveness, and blooms may return
- Typically proprietary blends of nontoxic dyes (WSDE 2016); most shading products are not labeled as registered pesticides, and full chemical composition may not be given with product
- Permit may be required
This aquatic growth control technology dates back at least 73 years (Eicher 1947), and commercial dye products for this purpose have been available for at least 40 years. Researchers have found that at least one shading dye does not significantly reduce visibility in water for swimmers and other recreators (Madsen et al. 1999). Dyes may be used in conjunction with other cyanobacteria preventive or control technologies. Perhaps most importantly, you might find that dyes have little to no effect on reducing cyanobacteria bloom frequency or severity. Some laboratory experiments and field-scale pilot studies conducted in 2- to 3-foot water depths showed that prescribed concentrations of a leading pond dye had little to no effect on algal
CASE STUDY EXAMPLES
Teton Pond, Dunbar, Nebraska, United States: Buglewicz and Hergenrader (1977) performed a field-scale pilot study on a 2.4-acre pond west of Dunbar, Nebraska, ~5.2 feet deep, and fed by a 147-acre watershed of fertilized farmland during the April to September growing season.
Six isolation test box enclosures were constructed within the pond. No dye was added to one box, which served as the test control, and no dye was added to the pond outside of the enclosures.
Alizanine blue dye was added to three enclosures at three different concentrations that reduced Secchi disc visibility from 10 feet to just 12, 6, and 4 inches, respectively. Secchi depths eventually stabilized to 12 inches in all blue-dyed boxes.
Sandolan dark brown dye was added to two enclosures at two different concentrations that reduced Secchi disc visibility from 10 feet to 24 and 12 inches, respectively. Secchi depths eventually stabilized to 18 inches in all brown-dyed boxes.
Cyanobacteria were eliminated from both Sandolan dark brown-dyed boxes and from one of the three enclosures dyed with Alizanine blue.
Cyanobacteria algal volumetric share increased substantially in the treated box, even though the cyanobacteria share remained steady in untreated control boxes.
Cyanobacteria treatment effectiveness results were mixed despite reducing light penetration. It is possible the test may not have fairly evaluated dyes as a preventive technology since cyanobacteria were already a sizable fraction of the total algal volume before the test was initiated.
COST ANALYSIS
Shading with dyes has a low seasonal cost for ponds or small lakes with limited flowthrough.
Relative cost per growing season: Shading with dyes (light filtering)
| ITEM | RELATIVE COST PER GROWING SEASON |
| Material | $$ |
| Equipment | $$ |
| Labor | $ |
| OVERALL | $$ |
REGULATORY AND POLICY CONSIDERATIONS
Commercially available, nontoxic dyes are suitable for uses in waters used for swimming and other recreational purposes; however, there may be regulatory obstacles or prohibitions against their use in drinking water reservoirs. A permit from the state herbicide or pesticide control agency may be required prior to use. Also, if the water body is a public water supply source, there may be federal, state, or local restrictions against use of shading dyes. Check with the environmental regulatory agency before moving ahead (NYSFOLA 2009).
The dyes will impart a new and unnatural color to the water that may not be appealing to some. Furthermore, the public may view the technique as adding a manmade “chemical” to the environment to engineer the disruption of a naturally occurring, albeit undesirable, aquatic phenomenon (NYSFOLA 2009). Before applying dyes to community waters, solicit input from stakeholders to ensure that there is public consensus for intervention.
REFERENCES
Boyd, Claude E., M. Hanapi, and M. Noor. 1982. “Aquashade(R) treatment of channel catfish ponds.” North American Journal of Fisheries Management 2 (2):193-196. doi: 10.1577/1548-8659(1982)2<193:atoccp>2.0.co;2.
Buglewicz, Eugene G., and Gary L. Hergenrader. 1977. “The impact of artificial reduction of light on a eutrophic farm pond.” Transactions of the Nebraska Academy of Sciences and Affiliated Societies 4.
Eicher, George. 1947. “Aniline dye in aquatic weed control.” The Journal of Wildlife Management 11 (3):193-197. doi: 10.2307/3796277.
Ludwig, Gerald M., Peter Perschbacher, and Regina Edziyie. 2010. “The effect of the dye Aquashade® on water quality, phytoplankton, zooplankton, and sunshine bass, Morone chrysops×M. saxatilis, fingerling production in fertilized culture ponds.” Journal of the World Aquaculture Society 41 (s1):40-48. doi: 10.1111/j.1749-7345.2009.00331.x.
Madsen, John D., Kurt D. Getsinger, R. Michael Stewart, John G. Skogerboe, David R. Honnell, and Chetta S. Owens. 1999. “Evaluation of transparency and light attenuation by Aquashade™.” Lake and Reservoir Management 15 (2):142-147. doi: 10.1080/07438149909353958.
NYSFOLA, (New York State Federation of Lake Associations). 2009. “Diet for a Small Lake: The Expanded Guide to New York State Lake and Watershed Management.” New York State Federation of Lake Associations, Inc. . https://nysfola.org/diet-for-a-small-lake/.
Spencer, David F. 1984. “Influence of Aquashade on growth, photosynthesis, and phosphorus uptake of microalgae.” Journal of Aquatic Plant Management 22:80-84.
Suski, J. G., C. M. Swan, C. J. Salice, and C. F. Wahl. 2018. “Effects of pond management on biodiversity patterns and community structure of zooplankton in urban environments.” Sci Total Environ 619-620:1441-1450. doi: 10.1016/j.scitotenv.2017.11.153.
USEPA. 2005. “Waste from the Production of Dyes and Pigments Listed as Hazardous EPA 530-F-05-004.” Washington, D. C. : U. S. Environmental Protection Agency. https://archive.epa.gov/epawaste/hazard/web/html/dyes-ffs.html.
WSDE. 2016. “Fact Sheet for the Aquatic Plant and Algae Management NPDES General Permit.” Olympia, WA: Washington State Department of Ecology. https://ecology.wa.gov/getattachment/c514c529-657d-425e-baf7-18591e4b4577/2016APAMFactSheet.pdf.
SKIMMING AND HARVESTING
In-lake Intervention Strategy
Limited Supporting Field Data
There is little available detail on the technologies for skimming or harvesting cyanobacteria from natural systems. A large surfactant, flotation, skimming, ozonation technology has been used in a pilot project in Florida (Page et al. 2020) that shows promise for removal of cyanobacteria and possible cyanotoxin destruction. A second is a skimming application in Southampton, New York (Southampton Press 2019). Although harvesters for submersed aquatic plants are known and used occasionally for removal of invasive submersed grasses, such as Hydrilla spp. (McGehee 1979), data that support the removal of cyanobacteria biomass from concentrated blooms with these techniques are limited.
EFFECTIVENESS
- Water body types: Pond, lake/reservoir, bay/estuary, systems allowing scum formation
- Any surface area or depth
- Water body uses: Any, except perhaps drinking water sources
- Any trophic state
- Any mixing regime
- Reported (no data) treatment of 100 M gal/day
NATURE OF HCB
- Scum-forming or floating HCBs
- Singular or repeating HCBs
- Toxic and nontoxic HCBs
- Intervention strategy
The sole estimate found for efficacy of harvesting and skimming is the 2019 Florida pilot project (Page et al. 2020). Unfortunately, much of the cyanobacteria present in this study were subsurface, and there was little cyanotoxin (microcystin) detected. Most results were for nutrient removal, but this method was quite effective in removing nitrogen and phosphorus, which are mostly found in the cyanobacteria biomass. There were two major limitations for the technology, however: huge capital and operations and management costs, as well as very high energy demand. Page et al. (2020) argue that these costs could be reduced if the harvested biomass could then be converted to biofuels and sold, but that technology is still in development.
As both skimming and flotation cannot remove an entire bloom, the remaining populations could re-seed and cause additional blooms. For skimmed cyanobacteria without biofuel conversion, the collected material could be considered hazardous waste with associated costs for its disposal. Subsequent disposal of harvested biomass may be limited depending on cyanotoxin content of the collected biomass.
ADVANTAGES
- Biofuel production from harvested biomass is proposed
- Low potential for adverse impacts
- High volumes of surface scum biomass can be harvested
LIMITATIONS
- Limited data
- Huge capital investment for reagents, air flotation technology, ozone, and operations and maintenance
- May not be scalable
- Hazardous waste designation for collected biomass possible
- Indiscriminate removal
- Scum is collected, so there may be surface water criteria concerns
- Unknown costs
- Need partners for post-collection commercial use
CASE STUDY EXAMPLES
Lake Okochobee and Newnans Lake, Florida, United States: Skimming alone or flotation followed by skimming are two strategies offered as intervention technologies for HCB removal in New York and Florida, respectively. In Florida (Page et al. 2020), a pilot study of the surfactant-flotation-skimming-ozone technology was conducted in Lake Okochobee and Newnans Lake. Unfortunately for estimates of efficacy, little scum cyanobacteria were present; most biomass was at depth and at low toxin concentrations (<1 ppb). Results indicated that most of the nitrogen and phosphorus was found in the cells, and removal of nutrients was very high. The technology shows promise, particularly when surface cyanobacteria densities are elevated, but treatment costs are very high, estimated from $2M to $18M per year.
Southampton, New York, United States: Detail on the pilot program in New York can be found in the Southampton Press (2019).
COST ANALYSIS
Costs are relatively low for simple skimming and very high for surfactant-flotation-skimming-ozone treatment. Specific equipment for skimming or harvesting would be required, and some form of power would be needed. The relative costs below are for skimming only and surfactant-flotation-skimming-ozonation. If harvested biomass can be processed for commercial uses, net overall cost may be reduced. Whether funds recovered from the sale of the collected material are passed on to the lake manager to reduce their costs is unknown.
Relative cost per growing season: Skimming and harvesting
| ITEM | RELATIVE COST PER GROWING SEASON |
| Material | $$–$$$ |
| Personal Protective Equipment | $$ |
| Equipment | $$–$$$ |
| Machinery | $$–$$$ |
| Tools | $–$$$ |
| Labor | $$–$$$ |
| O&M Costs | $–$$$ |
| Delivery | $$$ |
| OVERALL | $$–$$$ |
REGULATORY AND POLICY CONSIDERATIONS
Collected biomass may include intracellular cyanotoxins, so appropriate use of the harvested biomass will depend on the material’s cyanotoxin concentration. If nontoxic, landfill application or use as wet fertilizer might be possible; composting would likely be allowed, but authorities would need to be contacted for local regulations. If processors can be found for use in the synthesis of commercial products such as foam rubber or biofuels, disposal permitting may not be necessary. If cyanotoxins are present, then permits for use of the collected biomass would be needed, and local and state officials should be contacted. For both toxic and nontoxic biomass, harvesting would remove cellular nitrogen and phosphorus, assisting in nutrient removal from impacted water bodies and therefore possible “credit” for TMDLs in a watershed.
REFERENCES
McGehee, J. T. 1979. “Mechanical hydrilla control in Orange Lake, Florida.” J. Aquat. Plant Manage. 17:58-61.
Page, Martin A., Bruce A. MacAllister, Angela. Urban, Christopher L. Veinotte, Irene E. MacAllister, Kaytee L. Pokrzywinski, Jim. Riley, Edith Martinez-Guerra, Craig S. White, Chris. Grasso, Alan James Kennedy, Catherine C. Thomas, Justin. Billing, Andrew Schmidt, Dan. Levy, Bill. Colona, David. Pinelli, and Chandy. John. 2020. “Harmful Algal Bloom Interception, Treatment, and Transformation System, “HABITATS”, ERDC TR-20-1.” Champaign, IL: U. S. Army Corps or Engineers. Construction Engineering Research Laboratory. https://hdl.handle.net/11681/35214.
Southampton Press. 2019. “Governor Cuomo And DEC Launch Blue-Green Algae Removal Pilot Program In Southampton Village.” The Southampton Press, 10/3/2019, Environment/Neighborhood. https://www.27east.com/southampton-press/governor-cuomo-and-dec-launch-blue-green-algae-removal-pilot-program-in-southampton-village-1540878/.
ULTRASOUND
In-lake Intervention and Prevention Strategy
Limited/Emerging Supporting Field Data
Many species of cyanobacteria can regulate their buoyancy in the water column through special internal structures known as gas vesicles (Reynolds and Walsby 1975, Walsby et al. 1997). These provide a competitive advantage over other phytoplankton. Cells with gas vesicles accumulate at the surface during the day to use available light for photosynthesis, shading out competing non-cyanobacterial species. Late in the day, accumulated sugars or carbohydrates from daytime photosynthesis overcome the buoyancy from the gas vesicles and cells sink to cooler, nutrient-rich water, which allows them to continue to grow and maintain dominance. Not all cyanobacteria species appear to be capable of producing gas vesicles, and even among similar species, differences in relative abundance and activity of gas vesicles is evident (Brookes, Ganf, and Oliver 2000).
Disrupting the ability of cyanobacteria to maintain their position in the water column is a strategy employed by several cyanobacteria control methods. While some strategies do this by artificially mixing the water column (see artificial circulation and mechanical mixers strategy), others may bind the cells with flocculants to sink them out of the euphotic zone (see clay and surfactant flocculation technique). However, ultrasound generates acoustic cavitation bubbles (Wu, Joyce, and Mason 2012) and, through bubble collapse, targets these specific buoyancy control structures that are unique to cyanobacteria and a few other bacterial groups.
Ultrasound refers to a wide range of applications, so care must be taken to distinguish among technologies. Typically, ultrasonic generators produce a frequency (measured in megahertz, MHz), at a set power intensity (measured in watts per square centimeter), at a set duration (measured in time, typically minutes). High-power ultrasound is used to destroy bacteria and plankton in wastewater treatment (Wu and Mason 2017) and ship ballast (Holm et al. 2008). Ultrasonic technologies intended for cyanobacterial control use high-frequency sound waves to collapse gas vesicles (Rajasekhar et al. 2012).
The technology appears to have been used in the field first in the early 2000s (Lee, Nakano, and Matsumura 2002), though it was conceptualized earlier (Park et al. 2017). This exposure results in gas vesicle collapse but typically not complete lysis or degradation of the cell. Frequencies between 1.7 MHz and 20 kHz are typically used in some modulation (Hao et al. 2004), with reported durations ranging from few-second pulses to pulses of several hours . Effective removal of most HCB species appears to occur within 10 minutes of exposure under laboratory conditions, though there are limited field data to support this observation (Wu, Joyce, and Mason 2012, Park et al. 2017). At high energies, however, ultrasound may also disrupt colonies and even break cell walls, thus inhibiting growth (Lürling and Tolman 2014). Some laboratory testing shows destruction of the cyanotoxin microcystin (Liu et al. 2018, Song et al. 2005), probably by generation of free radicals (Joyce, Wu, and Mason 2010). However, the mechanisms responsible for these effects in laboratory settings may not apply directly to field application. Controlled laboratory conditions are rarely true to field conditions, where rainfall, water quality, water flow, turbulence, and water volume under sonic generators appear to play a vital factor in device performance (Park et al. 2017). Even under ideal conditions, energy transmission falls off quickly with increasing distance (Rajasekhar et al. 2012). Hence, the technique has limited range.
Under field conditions, effectiveness is thought to be dependent on generation of frequencies that match resonant frequencies of the gas vesicles (Rajasekhar et al. 2012). The few results are anecdotal with highly variable results. In a recent review, Lürling and Mucci (2020) conclude that low-frequency ultrasound should be avoided, as it is ineffective; high-frequency treatment is more effective, but it is costly due to energy demand, and its effective range is limited. Review of commercial claims on efficacy is difficult, as manufacturers consider technical specifications as proprietary information, making controlled, independent testing difficult. Studies that include technical details are rare and usually confined to laboratory conditions (Kong et al. 2019). Ultrasonic technologies are also not a short-term improvement technology, with many observed decreases or changes to ecological condition occurring over several weeks (Schneider, Weinrich, and Brezinski 2015, Villanueva et al. 2015). Off-target effects on other aquatic organisms, including zooplankton (Lürling and Tolman 2014), insects, and vertebrates such as fish, are possible, though documentation is limited.
EFFECTIVENESS
- Water body types: Pond, lake/reservoir
- Depth: Shallow to moderate
- Surface area: Small
- Any trophic state
- Any mixing regime, though mixed systems could result in less contact time
- Any water body use
NATURE OF HCB
- Effective on planktonic, gas-vesicle-containing cyanobacteria
- Toxic or nontoxic HCBs
- Other aquatic algae can be targeted
- Intervention strategy
ADVANTAGES
- Can move generators as needed and adjust frequency and length of exposure to target different species
- Some devices are coupled with real-time sensors to measure effectiveness
LIMITATIONS
- Highly variable results
- Does not appear to remove cyanotoxins
- If frequency causes cell lysis, extracellular cyanotoxin levels could increase
- Does not control nutrients
- Benthic blooms may still occur
- Expensive and proprietary constraints prevent inspection of conditions, frequencies, etc.
- High-power treatments can affect other organisms
- Limited by an effective radius for impact
CASE STUDY EXAMPLES
Reservoir, New Jersey, United States: Schneider, Weinrich, and Brezinski (2015) deployed a system of ultrasonic buoys in a 200-acre reservoir that historically had blooms with taste and odor issues. The reservoir had previously used copper as its primary treatment.
Reservoir 1 is a 200-acre water body with a mean depth of 17 feet. It is fed by a small brook and adjacent reservoir (Reservoir 2).
Four ultrasonic buoys were deployed in May 2014 to reduce total algae abundance and concentrations of taste and odor compounds. While total numbers of algae cells appeared to decline, it should be noted that copper applications were used along with the buoys. Also, technical specifications of the ultrasonic buoys (frequency and intensity) were not reported.
General levels of cyanobacteria increased during the monitoring period; however, a bloom of Aphanizomenon occurred once water from Reservoir 2 was allowed to flow into Reservoir 1 (August 13, 2014). A reduction in the bloom was not noted until September 17, 2015, and may have been due to either the length of exposure or the change in the ultrasound frequency to target Aphanizomenon spp.
COST ANALYSIS
Financial costs depend on site-specific geographical and lake morphology factors and water conditions. For example, for a large water body, multiple generators may be required to effectively prevent a bloom. The range and limitation, as well as the service and maintenance of each generator, must be factored into the cost of deploying this technology. As this is a preventive technology that does not address nutrient input, a backup treatment option should be planned for blooms of cyanobacterial species that do not form gas vesicles or are otherwise outside the treatment range of the technology.
Relative cost per growing season: Ultrasound
| ITEM | RELATIVE COST PER GROWING SEASON |
| Equipment | $$–$$$ |
| O&M Costs | $$–$$$ |
| OVERALL | $$–$$$ |
REGULATORY AND POLICY CONSIDERATIONS
Some generators can use solar panels for electricity, while others require shoreline tethering for power. Local permitting for installation and potential impacts to zooplankton and other aquatic life must be considered.
REFERENCES
Brookes, Justin D., George G. Ganf, and Roderick L. Oliver. 2000. “Heterogeneity of cyanobacterial gas-vesicle volume and metabolic activity.” Journal of Plankton Research 22 (8):1579-1589. doi: 10.1093/plankt/22.8.1579.
Hao, Hongwei, Minsheng Wu, Yifang Chen, Jiaowen Tang, and Qingyu Wu. 2004. “Cavitation Mechanism in Cyanobacterial Growth Inhibition by Ultrasonic Irradiation.” Colloids and Surfaces B: Biointerfaces 33:151-156. doi: 10.1016/j.colsurfb.2003.09.003.
Holm, E. R., D. M. Stamper, R. A. Brizzolara, L. Barnes, N. Deamer, and J. M. Burkholder. 2008. “Sonication of bacteria, phytoplankton and zooplankton: application to treatment of ballast water.” Mar Pollut Bull 56 (6):1201-8. doi: 10.1016/j.marpolbul.2008.02.007.
Joyce, E. M., X. Wu, and T. J. Mason. 2010. “Effect of ultrasonic frequency and power on algae suspensions.” J Environ Sci Health A Tox Hazard Subst Environ Eng 45 (7):863-6. doi: 10.1080/10934521003709065.
Kong, Y., Y. Peng, Z. Zhang, M. Zhang, Y. Zhou, and Z. Duan. 2019. “Removal of Microcystis aeruginosa by ultrasound: Inactivation mechanism and release of algal organic matter.” Ultrason Sonochem 56:447-457. doi: 10.1016/j.ultsonch.2019.04.017.
Lee, T. J., K. Nakano, and M. Matsumura. 2002. “A novel strategy for cyanobacterial bloom control by ultrasonic irradiation.” Water Sci Technol 46 (6-7):207-15.
Liu, Cheng, Zhen Cao, Siyuan He, Zhehao Sun, and Wei Chen. 2018. “The effects and mechanism of phycocyanin removal from water by high-frequency ultrasound treatment.” Ultrasonics Sonochemistry 41:303-309. doi: 10.1016/j.ultsonch.2017.09.051.
Lürling, M., and Y. Tolman. 2014. “Beating the blues: is there any music in fighting cyanobacteria with ultrasound?” Water Res 66:361-373. doi: 10.1016/j.watres.2014.08.043.
Lürling, Miquel, and Maíra Mucci. 2020. “Mitigating eutrophication nuisance: in-lake measures are becoming inevitable in eutrophic waters in the Netherlands.” Hydrobiologia. doi: 10.1007/s10750-020-04297-9.
Park, J., J. Church, Y. Son, K. T. Kim, and W. H. Lee. 2017. “Recent advances in ultrasonic treatment: Challenges and field applications for controlling harmful algal blooms (HABs).” Ultrason Sonochem 38:326-334. doi: 10.1016/j.ultsonch.2017.03.003.
Rajasekhar, Pradeep, Linhua Fan, Thang Nguyen, and Felicity Roddick. 2012. “A Review of the Use of Sonication to Control Cyanobacterial Blooms.” Water research 46:4319-29. doi: 10.1016/j.watres.2012.05.054.
Reynolds, C. S., and A. E. Walsby. 1975. “Water-Blooms.” Biological Reviews 50 (4):437-481. doi: 10.1111/j.1469-185X.1975.tb01060.x.
Schneider, Orren D., Lauren A. Weinrich, and Scott Brezinski. 2015. “Ultrasonic Treatment of Algae in a New Jersey Reservoir.” Journal – AWWA 107 (10):E533-E542. doi: 10.5942/jawwa.2015.107.0149.
Song, W., T. Teshiba, K. Rein, and K. E. O’Shea. 2005. “Ultrasonically induced degradation and detoxification of microcystin-LR (cyanobacterial toxin).” Environ Sci Technol 39 (16):6300-5. doi: 10.1021/es048350z.
Villanueva, Maria V., Maria C. Luna, Maria I. Gil, and Ana Allende. 2015. “Ultrasound treatments improve the microbiological quality of water reservoirs used for the irrigation of fresh produce.” Food Research International 75:140-147. doi: 10.1016/j.foodres.2015.05.040.
Walsby, Anthony E., Paul K. Hayes, Rolf Boje, and Lucas J. Stal. 1997. “The selective advantage of buoyancy provided by gas vesicles for planktonic cyanobacteria in the Baltic Sea.” New Phytologist 136 (3):407-417. doi: 10.1046/j.1469-8137.1997.00754.x.
Wu, X., E. M. Joyce, and T. J. Mason. 2012. “Evaluation of the mechanisms of the effect of ultrasound on Microcystis aeruginosa at different ultrasonic frequencies.” Water Res 46 (9):2851-8. doi: 10.1016/j.watres.2012.02.019.
Wu, Xiaoge, and Timothy Mason. 2017. “Evaluation of power ultrasonic effects on algae cells at a small pilot scale.” Water 9:470. doi: 10.3390/w9070470.
ULTRAVIOLET EXPOSURE
In-lake Intervention Strategy
Limited Supporting Field Data
Ultraviolet (UV) exposure is an advanced oxidation technique that is used most commonly to disinfect treated water in process-level treatments where this is a more established technique. UV exposure works by transferring electromagnetic energy from a UV bulb to stop organisms from reproducing by inactivating their DNA. UV-C light is typically used in treatment processes due to its short wavelength (190–280 nm). This type of UV light is highly energetic and has strong mutagenic effects on the DNA of most organisms (Pattanaik, Schumann, and Karsten 2007). UV exposure has been experimentally shown to oxidize microcystins and cylindrospermopsin but at extremely high doses that are not feasible for an in-field application (USEPA 2019). UV exposure has been shown to be effective at oxidizing cyanotoxins in production-scale treatment operations when used in tandem with a catalyst, such as hydrogen peroxide (Afzal et al. 2010).
Using UV light to control HCBs in surface waters is not an established technique. Some studies have shown experimentally that UV exposure can inhibit HCB growth (Alam et al. 2001, Sakai et al. 2007). While Alam et al. (2001) reported that boats equipped with UV lamps were used in several eutrophic lakes in Japan to control algal growth, detailed reports of field data could not be found. Currently, this approach is considered limited due to a lack of validated field applications specifically for HCBs. An alternative application strategy is the deployment of boats with UV lamps to stunt the growth of an active bloom. As reported indirectly in Alam et al. (2001), boats with UV lamps show some feasibility for smaller, shallow lakes. This approach has been piloted in Lake Tahoe to control invasive weeds (Tahoe Resource Conservation District 2018).
EFFECTIVENESS
- Water body type: Pond, lake/reservoir
- Surface area: Small
- Depth: Shallow
- Trophic state: Oligo-/mesotrophic
- Any mixing regime
- Water body use: Recreation, drinking water (treatment), treated wastewater/effluent
- Water bodies with low turbidity
NATURE OF HCB
- HCB types: Could be effective for planktonic or benthic blooms
- Repeating HCBs; useful for drinking water treatment in reservoirs and other source waters with chronic, recurring HCBs
- Approach is non-targeted; other microbes and photosynthetic organisms could be susceptible to DNA damage (Pattanaik, Schumann, and Karsten 2007)
- Potential as immediately effective strategy by suppressing cyanobacterial growth following exposure
- Intervention strategy
ADVANTAGES
- UV exposure (UV-C) has been shown to inhibit growth of Microcystis aeruginosa for several days in laboratory studies, even at relatively low doses (37 mW/s/cm-2)
- Technique does not directly produce waste by-products; there is some experimental evidence that UV exposures have the potential to photoconvert compounds such as pharmaceuticals (Canonica, Meunier, and von Gunten 2008)
LIMITATIONS
- UV exposures alone are not effective at oxidizing cyanotoxins, but they may be effective if used in tandem with a catalyst (for example, hydrogen peroxide)
- Limited peer-reviewed evidence to support the use of UV exposures to control algal or cyanobacterial growth in lakes
- Boat deployment requires depth-adjusted lamps to accommodate the vertical migration of cyanobacteria in the water column
- Effectiveness can be dampened by turbidity and dissolved organic carbon content in water body (Afzal et al. 2010)

Figure C-13. This boat built by Inventive Resources has a panel of UV lights that is lowered to expose aquatic invasive plants to UV light.
Source: Tahoe Daily Tribune 2017. Used with permission.
CASE STUDY EXAMPLES
Laboratory-scale: Tao et al. (2010) conducted a laboratory study comparing the effects of UV-C exposure on the growth of Microcystis aeruginosa and three green algae. UV-C exposures of 20–200 mJ/sq cm were shown to suppress the growth of M. aeruginosa for 3–13 days following a dose-dependent pattern.
Exposure to >100 mJ/sq cm resulted in the death of most exposed cells. Exposures ranging from 20 mJ/sq cm to 50 mJ/sq cm had sublethal effects. The three green algae did not experience significant effects across the 20–200 mJ/sq cm exposure range, suggesting that M. aeruginosa is more sensitive than other non-HCB taxa.
This suggests that UV-C treatment may be a relatively specific intervention strategy that may have minimal impact on other algae in the environment.
COST ANALYSIS
Large-scale use of UV treatment on a process scale is expensive, but it is less costly than other advanced oxidation and disinfection processes (Dore et al. 2013). There are few cost analyses for UV exposure via boat, specifically for management of HCBs. Labor costs for UV exposure for invasive weed control in Lake Tahoe were estimated at $28,000 for a UV-C treatment system 160 sq. feet across 1 acre (Tahoe Resource Conservation District 2018); capital costs were not provided.
Relative cost per growing season: UV exposure
| ITEM | RELATIVE COST PER GROWING SEASON |
| Material | $$$ |
| Personal Protective Equipment | $$ |
| Equipment | $$$ |
| Machinery | $$$ |
| Tools | $$$ |
| Labor | $$$ |
| O&M Costs | $$$ |
| OVERALL | $$$ |
REGULATORY AND POLICY CONSIDERATIONS
Using UV exposure to treat HCBs in the field may require permitting and reporting. From the Lake Tahoe example, permits were acquired from the Tahoe Regional Planning Agency, and an authorization letter was obtained from the U.S. Army Corps of Engineers. Other regulatory agencies (the Lahontan Regional Water Quality Control Board and California Department of Fish and Wildlife) were contacted and offered consent or requested incorporation of specific monitoring parameters (Tahoe Resource Conservation District 2018). Process-level applications for use of this technique would likely require permitting similar to the procedures above.
REFERENCES
Afzal, A., T. Oppenländer, J. R. Bolton, and M. G. El-Din. 2010. “Anatoxin-a degradation by advanced oxidation processes: vacuum-UV at 172 nm, photolysis using medium pressure UV and UV/H(2)O(2).” Water Res 44 (1):278-86. doi: 10.1016/j.watres.2009.09.021.
Alam, Z. B., M. Otaki, H. Furumai, and S. Ohgaki. 2001. “Direct and indirect inactivation of Microcystis aeruginosa by UV-radiation.” Water Res 35 (4):1008-14. doi: 10.1016/s0043-1354(00)00357-2.
Canonica, S., L. Meunier, and U. von Gunten. 2008. “Phototransformation of selected pharmaceuticals during UV treatment of drinking water.” Water Res 42 (1-2):121-8. doi: 10.1016/j.watres.2007.07.026.
Dore, Mohammaed H., Rajiv G. Singh, Arian Khaleghi-Moghadam, and Gopal Achari. 2013. “Cost differentials and scale for newer water treatment technologies.” International Journal of Water Resources ad environmental Engineering 5 (2):100-109.
Pattanaik, Bagmi, Rhena Schumann, and Ulf Karsten. 2007. “Effects of Ultraviolet Radiation on Cyanobacteria and their Protective Mechanisms.” In Algae and Cyanobacteria in Extreme Environments, edited by Joseph Seckbach, 29-45. Dordrecht: Springer Netherlands.
Sakai, Hiroshi, Kumiko Oguma, Hiroyuki Katayama, and Shinichiro Ohgaki. 2007. “Effects of low- or medium-pressure ultraviolet lamp irradiation on Microcystis aeruginosa and Anabaena variabilis.” Water Research 41 (1):11-18. doi: 10.1016/j.watres.2006.09.025.
Tahoe Resource Conservation District. 2018. Aquatic Invasive Plant Control Pilot Project Final Monitoring Report
Tao, Y., X. Zhang, D. W. Au, X. Mao, and K. Yuan. 2010. “The effects of sub-lethal UV-C irradiation on growth and cell integrity of cyanobacteria and green algae.” Chemosphere 78 (5):541-7. doi: 10.1016/j.chemosphere.2009.11.016.
USEPA. 2019. “Cyanobacteria and Cyanotoxins: Information for Drinking Water Systems.” U. S. Environmental Protection Agency. https://www.epa.gov/cyanohabs/health-effects-cyanotoxins.
C.2 COST COMPILATION FOR SEVERAL MITIGATION STRATEGIES
The Compilation of Costs table presents a compilation of costs in 2020 U.S. dollars for a suite of mitigation strategies. References marked with an asterisk are listed in USEPA (2015). For a summary of 31 individual oxygenation or aeration case studies, see Wagner (2015). Note: For ranges of costs/acre or costs/acre/year in the referenced citations, table data may represent averages for the ranges presented. NA = Not Available.
Compilation of costs (2020 U.S. dollars) for a suite of mitigation strategies
| LOCALE | WATER BODY | TREATMENT | CAPITAL COSTS ($/ACRE) | O&M COSTS ($/ACRE) | DURATION OF EFFECTIVENESS (DAYS) | REFERENCE |
| AERATION | ||||||
| FL | Lakes | Circulators | 385–4,527 | 116–2,182 | NA | Cooke et al. (2005) in Wagner (2015) |
| MA | Onota Lake (617 acres) | Deep-hole system | 700 | 91 | NA | Berkshire Regional Planning Commission (2004)* |
| MA | Lovers Lake and Stillwater Pond (55.5 acres) |
Hypolimnetic aeration | 1,904 | 106 | 15 | ENSR Corporation (2008)* |
| MA | Lovers Lake and Stillwater Pond (55.5 acres) |
Artificial circulation | 2,352 | 157 | 15 | ENSR Corporation (2008)* |
| MN | Twin Lake (20 acres) | Solar circulator hypolimnetic dispersal | 7,793 | 277 | 20 | Chandler (2013)* |
| MN | Twin Lake (20 acres) | Bottom bubbler | 12,992 | 1,939 | 20 | Chandler (2013)* |
| New England | Lakes (3) | Mechanical mixing | 10,000–50,000 per device | Requires power and mainte-nance | NEIWPCC (2015) | |
| NY | Lakes | Surface aeration (oxygenation and circulation) | 150–2,500 | NYDEC (2019) | ||
| NY | Lakes | Hypolimnetic aeration or oxygenation | >2,500 | NYDEC (2019) | ||
| USA | Lakes (33) | Circulators | 399 (>133-acre lake), 4,050 (<25-acre lake) | Cooke et al. (2005) in Wagner (2015) | ||
| USA? | Unknown lakes | Hypolimnetic aerators | 2,039 765 for large lakes | 10 | Cooke et al. (2005) in Wagner (2015) | |
| ALGAECIDES | ||||||
| New England | Lake surface blooms | Surface application | 100 | NEIWPCC (2015) | ||
| NY | Lakes | 5–25/acre-ft | <1 | NYDEC (2019) | ||
| ALUM TREATMENT | ||||||
| MA | Lovers Lake and Stillwater Pond (28.25 acres) | 9,026 | 0 | 15 | ENSR Corporation (2008)* | |
| MA | Morses Pond (104.5 acres) | Annual treatment 2008–2016 | 1,620 | 253 | 18 | Wagner (2017) |
| MN | Keller Lake (72 acres) | 914 | 0 | NA | Barr (2005)* | |
| MN | Kohlman Lake (74 acres) |
2,509 | 0 | NA | Barr (2005)* | |
| MN | Spring Lake (409 acres) |
2,837 | 0 | 10–32 | Barr (2005)* | |
| MN | Twin Lake (19 acres) | 8,624 | 0 | 1.5 | Chandler (2013)* | |
| New England | Lakes | Alum | 280–5,000 | Variable | NEIWPCC (2015) | |
| NY | Lakes | Alum or lanthanum-substituted clay | 100–500 | NYDEC (2019) | ||
| NY | Cossayuna Lake (35 acres) |
726 | 0 | NA | The LA Group (2001)* | |
| SD | Lake Mitchell (877 acres) | 4-year assumed whole-lake treatment | 247 | 0 | NA | Osgood (2002)* |
| WA | Green Lake (259 acres) | 10,241 | 0 | 10 | Herrera Environmental Consultants (2003)* | |
| WA | Lake Ketchum (25.5 acres) | Treating sediment | 8,736 | 0 | 4 | Burghdorff & Williams (2012)* |
| WA | Lake Ketchum (25.5 acres) | Treating water column | 1,614 | 0 | 1 | Burghdorff & Williams (2012)* |
| WA | Lake Lawrence (330 acres) | Alum+ monitoring | 3,350 | 619 (/month) | 20 | Tetra Tech (2004)* |
| WA | Lake Hicks (4 acres) | 13,690 | 0 | >10 | King County (2005)* | |
| WI | Cedar Lake (1,120 acres) | Alum twice/year, assume 1/2 lake | 2,200 | 0 | 10 | Cedar Lake Protection & Rehab District (2013)* |
| WI | E. Alaska Lake (41 acres) | 4,640 | 0 | NA | Hoyman (2011)* | |
| BARLEY STRAW | ||||||
| MD | Lake Williston (67 acres) | 500 bales + 3,200 labor | 85 | 0 | 1 | Calculated from Sellner et al. (2015) |
| MN | Twin Lake (20 acres) | 619 | 0 | NA | Chandler (2013)* | |
| New England | Lakes | 225 lbs/acre | 500 | 0 | Full season | NEIWPCC (2015) |
| BIOMANIPULATION | ||||||
| NY | Stocking piscivorous fish to control planktivorous fish | 100–2,000 | NYDEC (2019) | |||
| MN | Twin Lake (20 acres) | Removing, adding, and monitoring fish | 15,646 | 0 | NA | Chandler (2013)* |
| DREDGING | ||||||
| MA | Lovers Lake and Stillwater Pond (19 acres) | 91,100 | 0 | 10 | ENSR Corporation (2008)* | |
| MD | E. Lake Linganore (~100 acres) | 180,000 | 0 | NA | Bohnel (2019) | |
| MN | Keller Lake (72 acres) | 17,594 | 0 | NA | Barr (2005)* | |
| MN | Kohlman Lake (74 acres) |
26,375 | 0 | NA | Barr (2005)* | |
| MN | Twin Lake (20 acres) | 142,342 | 0 | NA | Chandler (2013)* | |
| NY | Cossayuna Lake (35 acres) |
29,305 | 0 | NA | The LA Group (2001)* | |
| WA | Lake Lawrence (330 acres) | 95,452 | 4,766 | 50 | Tetra Tech (2004)* | |
| CLAY AND SURFACTANT FLOCCULATION | ||||||
| CHN | Lake Tai | Kaolinite, soil | 148–245 | 0 | 148–245 | Pan et al. (2019) |
| CHN | Lake Tai | Kaolinite, soil + capping | 3,648–8,197 | 0 | 3,648–8,197 | Pan et al. (2019) |
| HERBICIDE TREATMENT | ||||||
| NY | Cossayuna Lake (35 acres) |
CuSO4 every year for 5 years | 933 | 0 | 1 | The LA Group (2001)* |
| HYDRAULIC MANIPULATION | ||||||
| MN | Twin Lake (20 acres) | 32,480 | 3,864 | 20 | Chandler (2013)* | |
| NY | Lakes | <10,000 | NYDEC (2019) | |||
| PEROXIDE | ||||||
| MD | Lake Anita Louise (5 acres) |
350 lbs granular H2O2 compound | 486 | 0 | >4 | Mattheiss, Sellner, and Ferrier (2017) |
| MD | Spahrs Quarry (7 acres) |
550 lbs granular H2O2 compound | 275 | 0 | NA | Campbell and Sellner (in preparation) |
| ULTRASOUND | ||||||
| New England | Lakes | 20kHz–1MHz sound waves | 2,400 | Some (power, mainte-nance) | Variable | NEIWPCC (2015) |
| NY | Lakes | 20kHz–1MHz sound waves | 5,000/unit | Some (power, mainte-nance) | NYDEC (2019) | |
C.3 ABRIDGED STRATEGIES
We necessarily limited this review to methods that are used in contemporary settings and have support from peer-reviewed literature. Some of the methods that were considered, but are not reviewed here, are briefly touched on below.
- Biochar: Proposed in several states; there are limited data to support its use for HCB prevention or intervention strategy. Biochar is believed to actively bind to minerals and nutrients. Some early reports indicate similar binding behavior to activated charcoal.
- Chlorine compounds in drinking water treatment: Chlorine is a common disinfectant used as a controlling substance for cyanobacteria in finished drinking water. However, its efficacy in open water systems remains unknown. The use of chlorination in drinking water plants reveals its reactivity and, thereby, possible future use in open waters.
- Electrochemical oxidation: This strategy pumps lake water through an anode that is surrounded by a steel cathode, effectively oxidizing cells and toxins. Powered by onboard generators, an array of these units are deployed near the water surface. Cyanobacteria and other phytoplankton, detritus, etc. are oxidized as the water is pumped through the tubes. The higher the voltage supplied, the shorter the exposure period needed. Pilot projects are currently underway with New York State Department of Environmental Conservation sponsorship.
- Nanobubbling: This technique creates <100 nanometer bubbles of ozone, oxygen, or air by pumping these gases through a perforated ceramic plate. The nanobubbles sink and persist for months, oxidizing organic matter in bottom sediments and in some non-replicated studies, reduces water column chlorophyll including cyanobacteria. Ongoing laboratory analyses have documented nanobubble-induced reductions in planktonic algae and cyanobacteria but more field trials with replicated sampling is required to ensure efficacy.
- Nitrogen addition: Proponents claim that adding nitrogen to alter the nitrogen–phosphorus ratio will disfavor the growth of cyanobacteria and favor other photosynthetic organisms. Eutrophication is a widespread problem, so adding nutrients is not considered to be a sustainable action.
- Permanganate: Permanganate is an oxidizing agent that has been used as an algaecide for in-lake treatment of HCBs and excessive algae levels, as well as mitigating cyanotoxins, in a limited number of documented cases during the past century. Permanganate may be applied by spraying water surfaces or by feeding solid or slurry forms from a watercraft. This strategy can be effective at both physically removing or damaging cyanobacterial cells and destroying cyanotoxins. Permanganate, when used as an open-water algaecide, is typically applied as a potassium permanganate product.
- Shade balls or floating covers: Proponents claim that these shading strategies, originally deployed to prevent evaporation and reduce light-facilitated chemical reactions, will also shade out cyanobacteria. While these methods may have limited application, they may not be practical for widespread use, especially in multipurpose water bodies.
- Weir curtains, barriers, and exclusion devices: Planktonic cyanobacteria can form thick surface scums, and the accumulations can be exacerbated by wind action, wave action, and reservoir discharge hydraulics. One strategy for mitigating the effect of a bloom is simply to physically exclude it. A barrier can be placed on or near the water surface to isolate and protect a high-value location, such as a swim beach or drinking water intake. While simple in principle, the concept has been difficult to implement and has not often been tested rigorously. The solution is probably not practical on a small scale, because engineering costs are high, but there are a few promising implementations in large drinking water reservoirs.
REFERENCES
Bohnel, Steve. 2019. “Lake Linganore dredging project to begin in next few months.” The Frederick News-Post, January 30, 2019. https://www.fredericknewspost.com/news/environment/lake-linganore-dredging-project-to-begin-in-next-few-months/article_27dc1bf6-37f2-5722-940e-1b4666c26080.html.
Campbell, S. , and K. G Sellner. in preparation. “Use of peroxide to control Planktothrix agardhii in a small quarry.”.
Cooke, G., E. Welch, S. Peterson, and S. Nichols. 2005. Restoration and Management of Lakes and Reservoirs. Boca Raton: CRC Press.
Mattheiss, J, K. G. Sellner, and D. Ferrier. 2017. “Lake Anita Louise Peroxide Treatment Summary December 2016.” https://www.lakelinganore.org/wp-content/uploads/2017/01/Peroxide-Application-Summary-Report-Final.pdf.
NEIWPCC. 2015. “Harmful Algal Bloom Control Method Synopses.” New England Interstate Water Pollution Control Commission. https://www.neiwpcc.org/neiwpcc_docs/NEIWPCC_HABControlMethodsSynopses_June2015.pdf.
NYDEC. 2019. “Harmful Algal Blooms (HABS) Program Guide, Version 3.” New York Department of Environmental Conservation. http://www.dec.ny.gov/docs/water_pdf/habsprogramguide.pdf.
Pan, G, X. Miao, L. Bi, H. Zhang, L. Wang, L. Wang, Z. Wang, J. Chen, J. Ali, M. Pan, J. Zhang, B. Yue, and T. Lyu. 2019. “Modified local soil (MLS) technology for harmful algal bloom control, sediment remediation, and ecological restoration.” Water 11. doi: 10.3390/w11061123.
Park, Chae-Hong, Myung-Hwan Park, Kim Keonhee, Nan-Young Kim, Young-Hyo Kim, Eun-Mi Gwon, Baik-Ho Kim, Byung-Jin Lim, and S. J. Hwang. 2017. “A Physical Pre-Treatment Method (Vertical Weir Curtain) for Mitigating Cyanobacteria and Some of Their Metabolites in a Drinking Water Reservoir.” Water 9:775. doi: 10.3390/w9100775.
Peters, Andrew 2017. “Technical Developments in Materials and Techniques for Fish and Debris Passage: The Iron Gate Dam Experience.” International Conference on Engineering and Ecohydrology for Fish Passage, Amherst, MA, June 21, 2017.sdasd
Sellner, Kevin, Allen Place, Ernest Williams, Yonghui Gao, Elizabeth VanDolah, Michael Paolisso, Holly Bowers, and Shannon Roche. 2015. “Hydraulics and Barley Straw (Hordeum vulgare) as effective Treatment Options for a Cyanotoxin-Impacted Lake.” International Conference on Harmful Algae.
USEPA. 2015. “A Compilation of Cost Data Associated with the Impaccts and Control of Nutrient Pollution EPA 820-F-15-096.” Washington D. C. : U. S. Environmental Protection Agency. https://www.epa.gov/sites/production/files/2015-04/documents/nutrient-economics-report-2015.pdf.
Wagner, Kenneth J. 2017. “Phosphorus inactivation of incoming storm water to reduce algal blooms and improve water clarity in an urban lake.” Lake and Reservoir Management 33 (2):187-197. doi: 10.1080/10402381.2017.1288669.
Wagner, Kenneth J. . 2015. “Oxygenation and Circulation to Aid Water Supply Reservoir Management.” Water Research Foundation. https://books.google.com/books/about/Oxygenation_and_Circulation_to_Aid_Water.html?id=hbwkrgEACAAJ.